LC-MS/MS method for quantifying the HIV-1 broadly neutralizing antibody PGT 121.414.LS in human serum
IF 2.8
3区 医学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
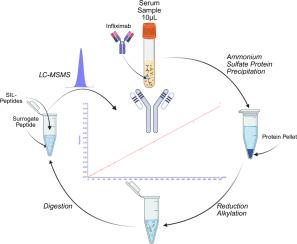
Quantitation of human immunodeficiency virus-1 (HIV-1) broadly neutralizing antibodies (bNAbs) in human serum is required for clinical trials investigating the pharmacokinetics, pharmacodynamics, and drug interactions of these treatments. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is gaining interest as an alternative to ligand binding for therapeutic antibody quantitation in serum. We report the validation of a method using nonspecific purification and targeted LC-MS/MS to quantify PGT 121.414.LS (a bNAb in development for HIV-1 prevention and treatment) in human serum. High-resolution spectra of tryptic peptides derived from the variable region were obtained on an Orbitrap for surrogate peptide selection, followed by multiple reaction monitoring using triple quadrupole mass spectrometry. Surrogate peptides were evaluated for linearity and reproducibility across the therapeutic concentration range using immunopurification or ammonium sulfate precipitation. Using ammonium sulfate precipitation, linear calibration curves were validated over 10–500 μg/mL (LLOQ at 10 μg/mL) using stable isotope labeled peptide internal standards. Method accuracy and reproducibility were evaluated using quality control samples (QCs) at four concentrations in the linear range. The average concentrations of all QCs fell within ICH M10 acceptance criteria. Matrix effects were investigated at the low and high QC concentrations across six lots of human serum. Dilutional integrity, stability, and effects of hemolysis were also assessed. The method exhibits minimal carryover and negligible crosstalk. The assay provides accurate quantification of PGT 121.414.LS in serum over the range of concentrations anticipated in specimens from treated persons living with HIV (PLWH) after initial dosing and prior to subsequent dosing of PGT 121.414.LS.
LC-MS/MS法定量测定HIV-1宽中和抗体PGT 121.414。人血清中的LS
人类免疫缺陷病毒-1 (HIV-1)广泛中和抗体(bNAbs)在人类血清中的定量是研究这些治疗的药代动力学、药效学和药物相互作用的临床试验所必需的。液相色谱-串联质谱(LC-MS/MS)作为一种替代配体结合的治疗性血清抗体定量方法越来越受到关注。我们报告了一种使用非特异性纯化和靶向LC-MS/MS定量PGT 121.414的方法的验证。LS(一种用于预防和治疗HIV-1的bNAb)在人血清中的应用。在Orbitrap上获得了来自可变区域的胰蛋白酶肽的高分辨率光谱,用于替代肽的选择,随后使用三重四极杆质谱法进行多重反应监测。采用免疫纯化或硫酸铵沉淀法评估替代肽在治疗浓度范围内的线性和可重复性。采用硫酸铵沉淀,在10 ~ 500 μg/mL (LLOQ为10 μg/mL)范围内用稳定同位素标记肽内标对线性校准曲线进行了验证。采用线性范围内4种浓度的质控样品(qc)评价方法的准确性和重现性。所有质谱仪的平均浓度均符合ICH M10验收标准。在6批人血清中研究了低、高QC浓度下的基质效应。稀释的完整性,稳定性和溶血效果也进行了评估。该方法具有最小的结转和可忽略的串扰。该检测提供了PGT 121.414的准确定量。在首次给药后和随后给药前,接受治疗的HIV感染者(PLWH)的血清中LS的浓度超出了预期范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chromatography B
医学-分析化学
CiteScore
5.60
自引率
3.30%
发文量
306
审稿时长
44 days
期刊介绍:
The Journal of Chromatography B publishes papers on developments in separation science relevant to biology and biomedical research including both fundamental advances and applications. Analytical techniques which may be considered include the various facets of chromatography, electrophoresis and related methods, affinity and immunoaffinity-based methodologies, hyphenated and other multi-dimensional techniques, and microanalytical approaches. The journal also considers articles reporting developments in sample preparation, detection techniques including mass spectrometry, and data handling and analysis.
Developments related to preparative separations for the isolation and purification of components of biological systems may be published, including chromatographic and electrophoretic methods, affinity separations, field flow fractionation and other preparative approaches.
Applications to the analysis of biological systems and samples will be considered when the analytical science contains a significant element of novelty, e.g. a new approach to the separation of a compound, novel combination of analytical techniques, or significantly improved analytical performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


