Sustainable upcycling of watermelon peels into a nanosensor for the selective detection of mefenamic acid, a nonsteroidal anti-inflammatory drug with antioxidant properties, in biofluids and pharmaceutical formulations
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
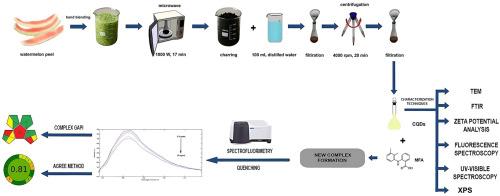
Mefenamic acid has recently been identified to possess antioxidant properties, expanding its potential applications beyond traditional pain relief. Emerging studies suggest its possible role in the prevention or management of Alzheimer’s disease through its anti-inflammatory and oxidative stress-reducing effects. This study represents an innovative, highly green eco-friendly method for determination of mefenamic acid in plasma, urine and in its pharmaceutical forms. It involves the transformation of waste watermelon peels into a highly fluorescent switch off nanosensor. For the first time, carbon quantum dots were synthesized from watermelon peel in one step via in situ microwave-assisted carbonization process, completed within 17.0 min without the addition of any external chemicals. The synthesized carbon quantum dots were found to have good fluorescence (λex/em 330.0/420.0 nm), high water solubility and good stability. The synthesized carbon dots were characterized using fluorescence spectroscopy, UV–Visible Spectroscopy, X-ray photoelectron spectroscopy, Zeta potential analysis; Transmission electron microscopy and Fourier transform infrared. The formed Carbon dots appeared to have average particle size of 3.69 - 6.19 nm and their shape was spherical. Mefenamic acid, a nonsteroidal anti-inflammatory anthranilic acid derivative with antioxidant properties, was determined using the synthesized carbon quantum dots by inner filter effect (IFE) and static fluorescence quenching mechanism. The quenching effect on the produced carbon quantum dots was utilized for determination of mefenamic acid over the range of (0.50 – 20.0 µg/mL). The % recovery of the developed method was found to be 100.03 ± 0.776 in pure form, 99.973 ± 0.713 in capsule, 99.70 ± 3.740 in plasma and 99.968 ± 4.005 in urine. Determination of mefenamic acid in the presence of different species indicated high selectivity of the suggested method. The validity of the proposed method was assessed according to the ICH recommendations. Different greenness assessment tools were used for evaluation of the proposed method sustainability. By using watermelon peel, typically regarded as waste, this method offers a green and sustainable route for synthesizing carbon quantum dots from natural sources. Also, using water as solvent without using any other organic solvents; it gives a great advantage to our work as it does not produce any chemical or harmful waste, which makes it safe for the analyst and earth.
西瓜皮的可持续升级回收成为一种纳米传感器,用于选择性检测生物流体和药物配方中的甲氧胺酸,甲氧胺酸是一种具有抗氧化特性的非甾体抗炎药
甲氧胺酸最近被确定具有抗氧化特性,扩大其潜在的应用超出传统的疼痛缓解。新出现的研究表明,它可能通过抗炎和减少氧化应激的作用,在预防或控制阿尔茨海默病方面发挥作用。这项研究代表了一种创新的、高度绿色环保的方法,用于测定血浆、尿液和其药物形式中的甲氧胺酸。它包括将废弃的西瓜皮转化为高荧光开关纳米传感器。首次以西瓜皮为原料,在不添加任何外部化学物质的情况下,采用原位微波辅助碳化工艺一步合成了碳量子点,在17.0 min内完成。合成的碳量子点具有良好的荧光(λex/em 330.0/420.0 nm)、高水溶性和良好的稳定性。采用荧光光谱、紫外-可见光谱、x射线光电子能谱、Zeta电位分析对合成的碳点进行了表征;透射电子显微镜和傅里叶变换红外。形成的碳点平均粒径为3.69 ~ 6.19 nm,形状为球形。甲氧胺酸是一种具有抗氧化性能的非甾体抗炎邻氨基苯酸衍生物,利用合成的碳量子点通过内滤效应(IFE)和静态荧光猝灭机制对其进行了测定。利用对碳量子点的猝灭效应,在(0.50 ~ 20.0µg/mL)范围内测定甲氧胺酸。方法的回收率分别为:纯品(100.03±0.776)、胶囊(99.973±0.713)、血浆(99.70±3.740)、尿液(99.968±4.005)。对不同种类甲芬那酸的测定结果表明,该方法具有较高的选择性。根据ICH的建议评估了所建议方法的有效性。采用不同的绿色评价工具对所提出方法的可持续性进行了评价。通过使用通常被视为废物的西瓜皮,该方法为从天然来源合成碳量子点提供了一条绿色和可持续的途径。同时,以水为溶剂,不使用任何其他有机溶剂;它给我们的工作带来了很大的优势,因为它不会产生任何化学物质或有害废物,这使得它对分析人员和地球都是安全的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


