[Zn1K2]Fe3(PO4)2(P2O7) as a Zn2+/K+ dual-ion cathode with enhanced diffusion kinetics and structural stability for aqueous zinc-ion batteries
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Aqueous zinc-ion batteries (AZIBs) have gained attention as safe and cost-effective alternatives to conventional lithium-ion batteries. However, their practical performance is often hindered by sluggish Zn2+ diffusion, primarily due to the large hydrated ionic radius and strong electrostatic interactions. To address this, we present a polyanion-based cathode material, [Zn1K2]Fe3(PO4)2(P2O7), that enables a reversible dual-ion intercalation mechanism involving both Zn2+ and K+. Compared to Zn2+, monovalent K+ interacts more weakly with the PO4/P2O7 framework and desolvates more readily at the electrolyte–cathode interface, enabling the Zn2+/K+ dual-ion system to achieve faster kinetics than the Zn2+-only system. This leads to significantly enhanced power capability and structural reversibility under the AZIB system. The discharge capacity of [Zn1K2]Fe3(PO4)2(P2O7) is 100.4 mAh g-1 at C/5 (1C = 100 mA g-1), corresponding to de/intercalation of 2 mol K+ and 0.5 mol Zn2+. Even at 5C, it maintains a capacity of 70 mAh g-1, outperforming the Zn2+-only analogue Zn2Fe3(PO4)2(P2O7) (55 mAh g-1). Galvanostatic intermittent titration technique measurements further confirm the enhanced ionic diffusion kinetics enabled by the Zn2+/K+ dual-ion system. Moreover, the capacity retention of [Zn1K2]Fe3(PO4)2(P2O7) is 84.6 % after 100 cycles, while Zn2Fe3(PO4)2(P2O7) deliver only 56.1 %. Advanced structural analyses reveal highly reversible lattice evolution during cycling, supporting the proposed dual-ion storage mechanism.
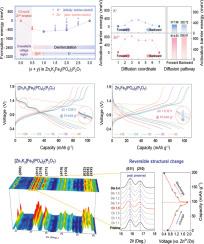
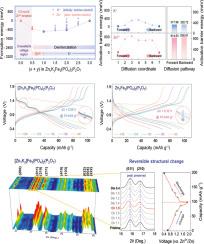
[Zn1K2]Fe3(PO4)2(P2O7)作为Zn2+/K+双离子阴极的扩散动力学和结构稳定性
水性锌离子电池(azib)作为传统锂离子电池的安全、经济的替代品而受到关注。然而,由于大的水合离子半径和强的静电相互作用,它们的实际性能经常受到Zn2+扩散缓慢的阻碍。为了解决这个问题,我们提出了一种多阴离子基阴极材料,[Zn1K2]Fe3(PO4)2(P2O7),它可以实现包括Zn2+和K+的可逆双离子插入机制。与Zn2+相比,一价K+与PO4/P2O7骨架的相互作用更弱,在电解质-阴极界面更容易脱溶,使得Zn2+/K+双离子体系比仅Zn2+体系实现更快的动力学。这导致AZIB系统下的动力能力和结构可逆性显著增强。[Zn1K2]Fe3(PO4)2(P2O7)在C/5 (1C = 100 mA g-1)下的放电容量为100.4 mAh g-1,对应于2 mol K+和0.5 mol Zn2+的脱插。即使在5C时,它也能保持70 mAh g-1的容量,优于仅含Zn2+的类似产品Zn2Fe3(PO4)2(P2O7) (55 mAh g-1)。恒流间歇滴定技术的测量进一步证实了Zn2+/K+双离子体系增强的离子扩散动力学。经过100次循环后,[Zn1K2]Fe3(PO4)2(P2O7)的容量保留率为84.6%,而Zn2Fe3(PO4)2(P2O7)的容量保留率仅为56.1%。先进的结构分析揭示了循环过程中高度可逆的晶格演化,支持了所提出的双离子储存机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


