An inducible model of human post-implantation development derived from primed and naive stem cells
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Early post-implantation human development is poorly understood due to limited access to natural embryos. Integrated stem cell-based embryo models (SCBEMs) offer an alternative, but current models face challenges in reproducibility, efficiency, and genomic stability. Here, we developed inducible SCBEMs (iSCBEMs) by combining primed human pluripotent stem cells (hPSCs) with transgene-induced extraembryonic cells derived from naive hPSCs. iSCBEMs recapitulate several key features of early post-implantation development, including amniotic-, yolk sac-, and chorionic-like cavity formation, differentiation of syncytiotrophoblast-like cells forming lacunae, bilaminar disk formation, anterior-posterior axis establishment, and early gastrulation. Single-cell RNA sequencing revealed that iSCBEMs recapitulate key cell types and developmental transitions characteristic of Carnegie stage 5–6 (CS5–CS6) embryos. We further traced the origins of amnion-, yolk sac endoderm-, and extraembryonic mesoderm-like cells, providing insights into their developmental trajectories. Although imperfect, human iSCBEMs represent a robust and valuable model for studying early post-implantation development, overcoming the limitations of natural embryo accessibility.
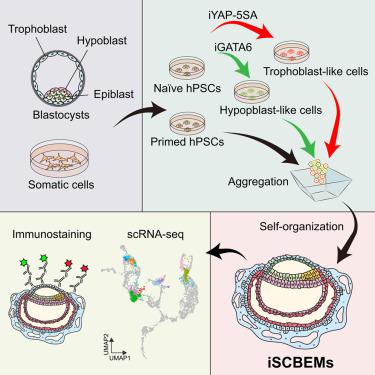
人类胚胎植入后发育的诱导模型来源于引物和原始干细胞
由于获得自然胚胎的机会有限,对早期植入后人类发育知之甚少。基于干细胞的集成胚胎模型(SCBEMs)提供了另一种选择,但目前的模型在可重复性、效率和基因组稳定性方面面临挑战。在这里,我们通过将引物的人多能干细胞(hPSCs)与来自原始hPSCs的转基因诱导的胚胎外细胞结合,开发了可诱导的SCBEMs (iSCBEMs)。isbems概括了胚胎着床后早期发育的几个关键特征,包括羊膜、卵黄囊和绒毛膜样腔的形成、合体滋养细胞样细胞的分化形成腔隙、双层盘的形成、前后轴的建立和早期原肠胚形成。单细胞RNA测序显示,iSCBEMs概括了卡内基5-6期(CS5-CS6)胚胎的关键细胞类型和发育转变特征。我们进一步追踪了羊膜、卵黄囊内胚层和胚外中胚层样细胞的起源,为它们的发育轨迹提供了见解。尽管尚不完善,但人类iSCBEMs克服了自然胚胎可及性的局限性,为研究胚胎着床后早期发育提供了一个强大而有价值的模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


