Unraveling B, N Co-doping synergy in graphitic carbon nitride for efficient CO2 cycloaddition with styrene oxide
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
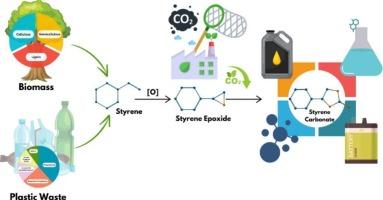
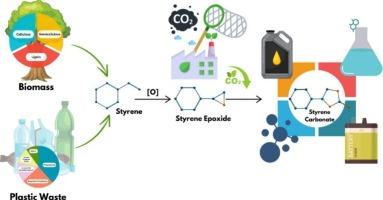
The cycloaddition of CO2 and styrene oxide to produce styrene carbonate under solvent-free conditions offers an eco-friendly, atom-efficient, and highly effective approach to CO2 utilization. This study presents a highly active and selective catalytic system based on boron and nitrogen co-doped graphitic carbon nitride (BN-GCN) for the efficient production of styrene carbonate. Unlike many conventional catalysts, the BN-GCN system operates without needing solvents or metals, functioning as a metal-free heterogeneous catalyst for the cycloaddition of CO2 with styrene oxide. It outperforms other heteroatom-doped graphitic carbon nitride (X-GCN) catalysts, with the enhanced activity attributed to the synergistic interaction of reactants with B and N sites. XPS analysis reveals that the N moiety and the B atom are critical active sites for cooperative catalysis. The BN-GCN catalyst achieves 75 % conversion and > 90% selectivity for styrene carbonate (SC) formation under mild conditions (353 K, 2 bar CO2) within 3 h. Comprehensive material characterization, kinetic studies, and reaction data confirm that B and N co-doping facilitates the co-activation of CO2 and epoxide. Based on density functional theory (DFT) calculations, and experiments, a convenient mechanism has been proposed for this unique method. The B–N bond duality in BN-GCN offers a cost-effective, sustainable, and efficient catalytic pathway for CO2 utilization.
揭示B, N共掺杂在石墨氮化碳与苯乙烯氧化物的高效CO2环加成中的协同作用
在无溶剂条件下,二氧化碳和氧化苯乙烯环加成生成碳酸苯乙烯是一种环保、原子高效、高效的二氧化碳利用方法。提出了一种基于硼氮共掺杂石墨氮化碳(BN-GCN)的高活性选择性催化体系,用于高效制备碳酸苯乙烯。与许多传统催化剂不同,BN-GCN系统不需要溶剂或金属,作为一种无金属的非均相催化剂,用于二氧化碳与苯乙烯氧化物的环加成。它优于其他杂原子掺杂石墨氮化碳(X-GCN)催化剂,其活性增强归因于反应物与B和N位点的协同相互作用。XPS分析表明,N部分和B原子是协同催化的关键活性位点。BN-GCN催化剂在温和条件下(353 K, 2 bar CO2)在3 h内生成碳酸苯乙烯(SC),转化率为75% %,选择性为 >; 90%。综合材料表征、动力学研究和反应数据证实,B和N共掺杂有利于CO2和环氧化物的共活化。基于密度泛函理论(DFT)的计算和实验,提出了一种简便的机制。BN-GCN中B-N键的二元性为CO2利用提供了一种经济、可持续、高效的催化途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


