Single-cell atlas of human liver and blood immune cells across fatty liver disease stages reveals distinct signatures linked to liver dysfunction and fibrogenesis
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
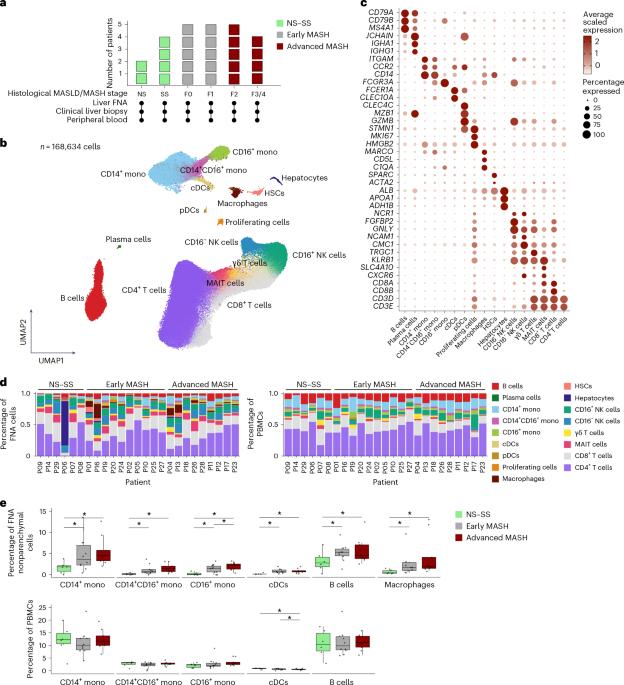
Immune cells play a central yet poorly understood role in metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis (MASLD/MASH), a global cause of liver disease with limited treatment. Limited access to human livers and lack of studies across MASLD/MASH stages thwart identification of stage-specific immunological targets. Here we provide a unique single-cell RNA sequencing atlas of paired peripheral blood and liver fine-needle aspirates from a full-spectrum MASLD/MASH human cohort. Our findings included heightened immunoregulatory programs with MASH progression, such as enriched hepatic regulatory T cells, monocytic myeloid-derived suppressor cells, TREM2+S100A9+ macrophages and S100hiHLAlo type 2 conventional dendritic cells. Hepatic cytotoxic T cell functions increased with inflammation, but decreased with fibrosis, while acquiring an exhausted signature, whereas natural killer cell-driven toxicity intensified. Our dataset proposes immunological mechanisms for increased fibrogenesis and vulnerability to liver cancer and infections in MASH and provides a basis for a deeper understanding of human immunological dysfunction in chronic liver disease and a roadmap to new targeted therapies. Alatrakchi and colleagues profile immune cells from liver and blood obtained from patients with MASLD/MASH using single-cell sequencing. They note increased immunoregulatory programs that correlated with increased fibrogenesis and disease progression.
人类肝脏和血液免疫细胞在脂肪肝疾病阶段的单细胞图谱揭示了与肝功能障碍和纤维化有关的独特特征
免疫细胞在代谢功能障碍相关的脂肪性肝病和代谢功能障碍相关的脂肪性肝炎(MASLD/MASH)中发挥着核心但鲜为人知的作用,这是一种治疗有限的全球肝病病因。对人类肝脏的有限获取和缺乏对MASLD/MASH分期的研究阻碍了对分期特异性免疫靶点的识别。在这里,我们提供了来自全谱MASLD/MASH人类队列的配对外周血和肝脏细针抽吸物的独特单细胞RNA测序图谱。我们的研究结果包括随着MASH进展而增强的免疫调节程序,如肝脏调节性T细胞、单核髓源性抑制细胞、TREM2+S100A9+巨噬细胞和S100hiHLAlo 2型常规树突状细胞的富集。肝细胞毒性T细胞功能随着炎症而增加,但随着纤维化而下降,同时获得耗尽的特征,而自然杀伤细胞驱动的毒性则增强。我们的数据集提出了MASH中纤维化增加、肝癌易感性和感染的免疫学机制,并为更深入地了解慢性肝病中的人类免疫功能障碍和新的靶向治疗路线图提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


