Drug delivery, development, and technological aspects for peripheral drug eluting stents
IF 17.6
1区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Drug-eluting stents are the standard therapy for arterial occlusions, particularly in peripheral arterial disease, owing to their efficacy in mitigating in-stent restenosis, maintaining favorable biocompatibility, and improving patient compliance. Their performance can be enhanced through the integration of particulate systems, cytostatic agents, and biodegradable polymers. The complexities associated with chronic disease progression, recurrent in-stent restenosis, the impracticality of long-term animal studies, and the absence of United States Food and Drug Administration-endorsed in vitro drug release protocols for peripheral drug-eluting stents underscore the need for modified strategies and accelerated in vitro release testing as a quality control strategy. In addition to in vitro drug release, other critical evaluation parameters for coated stents include coating uniformity, thickness, drug content, biodegradability, particulate matter, and sterility testing. Ethylene oxide is the most widely used method for the sterilization of drug-eluting stents. Despite their clinical significance, standardized regulatory guidelines and a unified scientific framework for stability testing remain limited. This review provides a comprehensive overview of drug delivery strategies for peripheral drug-eluting stents, coating methodologies, evaluation criteria, in vitro drug release and permeation studies, preclinical animal models, drug release correlations, and stability considerations, along with perspectives on future advancements and opportunities in this field.
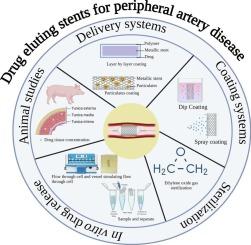
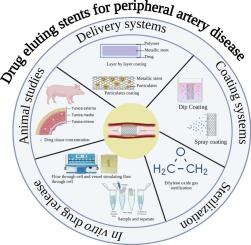
外周药物洗脱支架的药物输送、开发和技术方面
药物洗脱支架是动脉闭塞的标准治疗方法,特别是外周动脉疾病,因为它们在减轻支架内再狭窄、保持良好的生物相容性和提高患者依从性方面的疗效。它们的性能可以通过颗粒系统、细胞抑制剂和可生物降解聚合物的整合来增强。慢性疾病进展、复发性支架内再狭窄、长期动物研究的不可行性以及缺乏美国食品和药物管理局批准的外周药物洗脱支架体外药物释放方案的复杂性,强调了将改进策略和加速体外药物释放测试作为质量控制策略的必要性。除了体外药物释放外,涂层支架的其他关键评价参数包括涂层均匀性、厚度、药物含量、生物降解性、颗粒物和无菌性测试。环氧乙烷是目前应用最广泛的药物洗脱支架灭菌方法。尽管具有临床意义,但标准化的监管指南和统一的稳定性测试科学框架仍然有限。本文综述了外周药物洗脱支架的药物递送策略、涂层方法、评估标准、体外药物释放和渗透研究、临床前动物模型、药物释放相关性和稳定性考虑,以及对该领域未来进展和机遇的展望。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
28.10
自引率
5.00%
发文量
294
审稿时长
15.1 weeks
期刊介绍:
The aim of the Journal is to provide a forum for the critical analysis of advanced drug and gene delivery systems and their applications in human and veterinary medicine. The Journal has a broad scope, covering the key issues for effective drug and gene delivery, from administration to site-specific delivery.
In general, the Journal publishes review articles in a Theme Issue format. Each Theme Issue provides a comprehensive and critical examination of current and emerging research on the design and development of advanced drug and gene delivery systems and their application to experimental and clinical therapeutics. The goal is to illustrate the pivotal role of a multidisciplinary approach to modern drug delivery, encompassing the application of sound biological and physicochemical principles to the engineering of drug delivery systems to meet the therapeutic need at hand. Importantly the Editorial Team of ADDR asks that the authors effectively window the extensive volume of literature, pick the important contributions and explain their importance, produce a forward looking identification of the challenges facing the field and produce a Conclusions section with expert recommendations to address the issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


