Syngas production for steelmaking applications via dry reforming of methane using rare earth-containing aerogel catalysts: Evaluation of resistance to deactivation by coke deposition
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
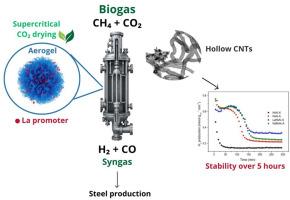
This study investigates the performance of Ni-Al2O3 aerogel catalysts, both unpromoted and promoted with rare earth oxides (La and Nd), in the dry reforming of methane for syngas production, with a focus on their application in the direct reduction of iron oxides. Additionally, the catalyst synthesis offers further advantages during the aerogel drying step, owing to the use of CO2 as the drying medium. For comparison purposes, a Ni-Al2O3 xerogel was also prepared and characterized to highlight the benefits of supercritical drying in aerogel synthesis. The influence of reaction temperature (600–800 °C) and rare earth promotion (La, Nd) on CH4 and CO2 conversion, H2 and CO production, and carbon deposition was investigated using a gaseous mixture containing CH4/CO2 (molar ratio 1:1). CH4 and CO2 conversions increased while carbon deposition decreased with the increase in temperature. Reverse water-gas shift, reverse Boudouard reaction, and CO reduction were the primary parallel reactions observed. The addition of rare earth promoters further improved catalyst performance, particularly La-promoted catalysts, which exhibited higher activity (0.34 and 0.67 mmol gcat−1 min−1 for CH4 and CO2, respectively). Carbon deposition profile was monitored along the reaction time, and all catalysts tested showed a net zero carbon deposition rate after 24 h of operation. Deactivation was not measured due to the hollow morphology of the deposited carbon nanotubes. The H2/CO ratio (around 0.3–0.5) of the produced synthesis gas makes it suitable for iron reduction in steelmaking.
用含稀土气凝胶催化剂通过甲烷干重整生产炼钢合成气:焦炭沉积的抗失活性评价
本研究研究了Ni-Al2O3气凝胶催化剂的性能,包括未促进和稀土氧化物(La和Nd)促进,在甲烷干重整合成气生产中的性能,重点研究了它们在直接还原氧化铁中的应用。此外,由于使用CO2作为干燥介质,催化剂合成在气凝胶干燥步骤中提供了进一步的优势。为了比较起见,还制备了Ni-Al2O3气凝胶,并对其进行了表征,以突出超临界干燥在气凝胶合成中的优势。研究了反应温度(600 ~ 800℃)和稀土促进剂(La、Nd)对CH4和CO2转化、H2和CO生成以及碳沉积的影响。随着温度的升高,CH4和CO2转化率增加,碳沉积减少。观察到的主要平行反应为逆水气转换、逆Boudouard反应和CO还原反应。稀土促进剂的加入进一步提高了催化剂的性能,尤其是la促进剂,对CH4和CO2的催化活性分别为0.34和0.67 mmol gcat−1 min−1。在整个反应过程中监测了碳沉积情况,所有测试的催化剂在运行24 h后均显示净碳沉积率为零。由于沉积的碳纳米管的空心形貌,没有测量失活。生产的合成气H2/CO比(约0.3-0.5)适合炼钢中铁的还原。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


