Bifunctional adECM bioscaffold with STIM1-ASCs and IGF-2 promotes functional masseter VML repair via myogenesis and fibrosis suppression
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Craniofacial muscles are essential for a variety of functions, including fine facial expressions. Severe injuries to these muscles often lead to more devastating consequences than limb muscle injuries, resulting in the loss of critical functions such as mastication and eyelid closure, as well as facial aesthetic impairment. Therefore, the development of targeted repair strategies for craniofacial muscle injuries is crucial. In this study, we engineered an adipose-derived decellularized extracellular matrix (adECM) bioscaffold co-loaded with seed cells and bioactive factors. The seed cells were STIM1-overexpressing adipose-derived stem cells (STIM1-ASCs), which exhibit directed and highly efficient myogenic differentiation, addressing the low differentiation efficiency of conventional ASCs that limits muscle regeneration. The bioactive factor used was insulin-like growth factor-2 (IGF-2), which modulates the immune microenvironment by reprogramming mitochondrial energy metabolism to promote M2 macrophage polarization. These M2 macrophages further suppress fibroblast collagen deposition, alleviating muscle fibrosis, while simultaneously enhancing the myogenic differentiation of STIM1-ASCs and myotube formation. Together, the recellularized adECM bioscaffold harnesses these dual mechanisms (promoting functional muscle regeneration and anti-fibrotic repair) to significantly improve the recovery of volumetric muscle loss (VML) in the masseter. The development of this bifunctional bioscaffold offers a novel therapeutic strategy and theoretical foundation for treating severe craniofacial muscle injuries.
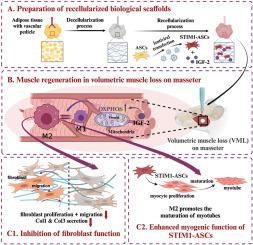
含有STIM1-ASCs和IGF-2的双功能adECM生物支架通过肌肉发生和纤维化抑制促进功能性咬肌VML修复
颅面肌对多种功能至关重要,包括精细的面部表情。这些肌肉的严重损伤往往会导致比肢体肌肉损伤更严重的后果,导致咀嚼和眼睑关闭等关键功能的丧失,以及面部审美障碍。因此,颅面肌损伤的靶向修复策略的发展是至关重要的。在这项研究中,我们设计了一种脂肪来源的脱细胞细胞外基质(adECM)生物支架,共负载种子细胞和生物活性因子。种子细胞是stim1过表达的脂肪来源干细胞(STIM1-ASCs),表现出定向和高效的肌源性分化,解决了传统ASCs低分化效率限制肌肉再生的问题。使用的生物活性因子是胰岛素样生长因子-2 (IGF-2),它通过重编程线粒体能量代谢来调节免疫微环境,促进M2巨噬细胞极化。这些M2巨噬细胞进一步抑制成纤维细胞胶原沉积,减轻肌肉纤维化,同时增强STIM1-ASCs的成肌分化和肌管形成。总之,再细胞化的adECM生物支架利用这两种机制(促进功能性肌肉再生和抗纤维化修复)来显著改善咬肌体积性肌肉损失(VML)的恢复。这种双功能生物支架的开发为治疗严重颅面肌肉损伤提供了新的治疗策略和理论基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


