Synthesis and antifungal activity of polyalthic acid-1,2,3-triazole-arylsulfonamide derivatives
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The increasing number of fungal infections and resistance to available drugs represent a serious threat to public health. In this context, the aim of this work was the synthesis of a novel series of polyalthic acid-1,2,3-triazole-arylsulfonamide derivatives by Cu(I)-catalysed azide-alkyne cycloaddition reactions (“click chemistry”), along with the evaluation of their antifungal properties against three Candida species. The best activities were observed for compounds 4, 6 and 7 against Candida albicans (MIC/MFC 6.25 to 12.5 μg/ml), and for compounds 9 and 3 against Candida krusei (MIC/ MFC 12.5 to 50 μg/ml). These findings highlight the potential of polyalthic acid derivatives as viable candidates for the development of new triazole antifungal agents.
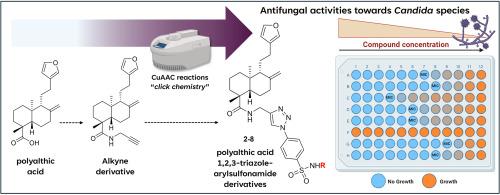
聚醛酸-1,2,3-三唑芳基磺酰胺衍生物的合成及抑菌活性研究
真菌感染数量的增加和对现有药物的耐药性对公众健康构成严重威胁。在此背景下,本研究的目的是通过Cu(I)催化叠氮-炔环加成反应(click chemistry)合成一系列新的聚醛酸-1,2,3-三唑-芳基磺酰胺衍生物,并评价其对三种念珠菌的抗真菌性能。化合物4、6和7对白色念珠菌(MIC/MFC为6.25 ~ 12.5 μg/ml)和化合物9和3对克鲁氏念珠菌(MIC/MFC为12.5 ~ 50 μg/ml)的活性最强。这些发现突出了多醇酸衍生物作为开发新的三唑类抗真菌药物的可行候选者的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


