Synthesis of active pharmaceutical ingredient atomoxetine via desulfurative halogenation
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An innovative preparation of the Active Pharmaceutical Ingredient (API) Atomoxetine has been developed. Key advantages of the synthetic procedure include: a one-pot preparation of a 1,3-bis-electrophilic phenyl-propionic synthon obtained via desulfurative chlorination of an easy to make thiophenyl sulfide; an unreported strategy for the preparation of secondary amines. The synthesis involves a total of four consecutive steps from unexpensive and readily available reagents and employes mild conditions.
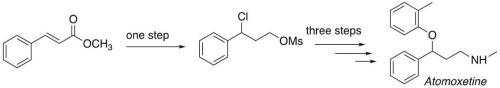
活性药物成分托莫西汀的脱硫卤化合成
研制了一种新颖的活性药物成分(API)阿托莫西汀制剂。该合成方法的主要优点包括:一锅制备1,3-二亲电苯-丙酸合成物,通过脱硫氯化得到易于制备的硫代苯基硫化物;一种未报道的制备仲胺的方法。该合成包括四个连续的步骤,从便宜和容易获得的试剂和雇员温和的条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


