Vibronic coupling shapes circularly polarized luminescence and quantum yields of carbo [6]helicene and bora [6]helicene
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
Theoretical guidance for the development of high-performance fluorophores based on accurate predictions of spectral properties and fluorescence quantum yields would be highly beneficial for the purposeful design of new fluorophores. This work presents a theoretical investigation into bora[6]helicene and its designed carbo[6]helicene counterpart to understand the impact of substituting a carbon substituent for a boron substituent on optical properties and quantum efficiencies. The differences in electronic characteristics between bora[6]helicene and carbo[6]helicene, as well as the effects of carbon substitution on spectral properties, were analyzed. Here, we applied the time-dependent correlation function approaches to calculate the transition nature, circularly polarized luminescence (CPL), internal conversion (IC), and intersystem crossing (ISC) rates of these two molecules. The results indicate that bora[6]helicene and carbo[6]helicene exhibit entirely distinct electronic properties with different HOMO-LUMO gaps. Theoretical vibronic spectroscopy results further confirm that carbon substitution significantly affects chiroptical properties, including spectral shape, width, intensity, and wavelength. To further characterize the excited states, radiative and non-radiative rates were calculated to predict fluorescence quantum yields (QYs). And we analyze the effect of different broadening functions (Gaussian, Lorentzian, or Voigt) on QYs. The calculated Voigt broadening function QYs of bora[6]helicene is consistent with the experimental value, confirming the reliability of our model. Compared with the theoretically predicted carbo[6]helicene, the QYs of bora[6]helicene is significantly larger. This work proves that compared with a carbon atom, the [6]helicene doped with a boron atom significantly improves the fluorescence quantum yield.
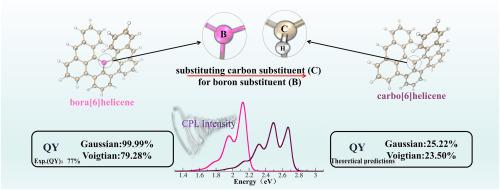
振动耦合影响了碳[6]螺旋烯和硼[6]螺旋烯的圆偏振发光和量子产率
基于对光谱特性和荧光量子产率的准确预测,为开发高性能荧光团提供理论指导,将对有目的地设计新的荧光团非常有益。本文对硼[6]螺旋烯及其设计的碳[6]螺旋烯进行了理论研究,以了解用碳取代基取代硼取代基对光学性质和量子效率的影响。分析了硼[6]螺旋烯与碳[6]螺旋烯电子特性的差异,以及碳取代对其光谱特性的影响。在这里,我们应用时间相关函数方法来计算这两个分子的跃迁性质、圆偏振发光(CPL)、内部转换(IC)和系统间交叉(ISC)速率。结果表明,硼[6]螺旋烯和碳[6]螺旋烯在HOMO-LUMO间隙不同时表现出完全不同的电子性质。理论振动光谱学结果进一步证实了碳取代对光谱形状、宽度、强度和波长等热学性质的显著影响。为了进一步表征激发态,计算了辐射率和非辐射率来预测荧光量子产率(QYs)。我们还分析了不同的展宽函数(高斯函数、洛伦兹函数和Voigt函数)对QYs的影响。计算得到的bora[6]螺旋烯的Voigt展宽函数QYs与实验值吻合,证实了模型的可靠性。与理论预测的碳[6]螺旋烯相比,硼[6]螺旋烯的QYs显著增大。本工作证明,与碳原子相比,掺杂硼原子的[6]螺旋烯显著提高了荧光量子产率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


