Exploring the potential antibacterial mechanism of the goose eggshell-derived CaO nanoparticles for deactivation of pharmaceutical wastages and bacteria
Q3 Materials Science
引用次数: 0
Abstract
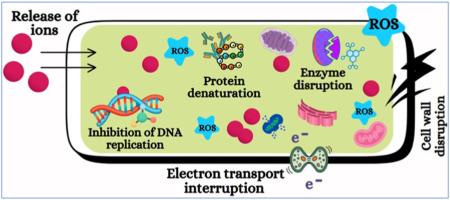
Environmental contamination caused by pharmaceutical residues and textile dye effluents poses significant challenges due to their chemical stability, toxicity, and resistance to conventional treatment methods. This study investigates the green synthesis of calcium oxide (CaO) NPS derived from goose eggshells via thermal decomposition at 900 °C, and evaluates their efficacy in photocatalytic degradation and antibacterial applications. Comprehensive characterization using XRD, FTIR, FESEM, UV–Vis studies confirmed the successful formation of phase-pure, highly crystalline CaO NPS with an average particle size of 47.9 nm and a direct optical bandgap of 3.41 eV. The photocatalytic performance of the synthesized CaO Nanoparticles (NPs) was assessed through the degradation of Safranin dye (a model cationic dye) and the pharmaceutical compound Paracetamol under natural sunlight. The NPS achieved degradation efficiencies of 97.43 % for Safranin and 91.25 % for Paracetamol, following pseudo-first-order kinetics. The degradation rate constant for Safranin (2.896 × 10−2 min−1) was higher than that for Paracetamol (1.551 × 10−2 min−1), likely due to more favourable adsorption and stronger electrostatic interactions between the cationic dye molecules and the negatively charged CaO surface. In addition to their photocatalytic properties, the CaO NPS demonstrated significant antibacterial activity, particularly against Gram-positive Staphylococcus aureus, with a maximum zone of inhibition of 19.4 mm. The enhanced antibacterial performance is attributed to the nanoscale size, high surface reactivity, and the alkaline nature of CaO, which collectively disrupt bacterial membrane integrity. Overall, this work underscores the potential of bio-waste-derived CaO NPS as an environmentally sustainable, cost-effective, and multifunctional material for the dual purpose of wastewater remediation and microbial control.
探讨鹅蛋壳制备的氧化钙纳米颗粒对药物废物和细菌失活的潜在抑菌机制
由于药物残留和纺织染料废水的化学稳定性、毒性和对常规处理方法的抗性,它们对环境污染构成了重大挑战。本研究以鹅蛋壳为原料,在900℃下进行热分解绿色合成氧化钙(CaO) NPS,并评价其光催化降解和抗菌性能。通过XRD、FTIR、FESEM、UV-Vis等综合表征,成功制备出相纯、高结晶的CaO NPS,平均粒径为47.9 nm,直接光学带隙为3.41 eV。通过在自然光照下对红色素(一种模型阳离子染料)和药物化合物扑热息痛的降解,评价了合成的CaO纳米颗粒(NPs)的光催化性能。NPS对红花素的降解效率为97.43%,对扑热息痛的降解效率为91.25%,符合准一级动力学。红花素的降解速率常数为2.896 × 10−2 min−1,高于扑热息痛的降解速率常数(1.551 × 10−2 min−1),这可能是由于阳离子染料分子与带负电的CaO表面之间具有更有利的吸附和更强的静电相互作用。除了光催化性能外,CaO NPS还表现出显著的抗菌活性,特别是对革兰氏阳性金黄色葡萄球菌,其最大抑制区为19.4 mm。增强的抗菌性能归因于CaO的纳米级尺寸、高表面反应性和碱性,这些因素共同破坏了细菌膜的完整性。总的来说,这项工作强调了生物废物来源的CaO NPS作为一种环境可持续、成本效益高的多功能材料的潜力,可用于废水修复和微生物控制的双重目的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JCIS open
Physical and Theoretical Chemistry, Colloid and Surface Chemistry, Surfaces, Coatings and Films
CiteScore
4.10
自引率
0.00%
发文量
0
审稿时长
36 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


