dre-miR-223/IKKα/NF-κB promotes inflammatory response in zebrafish liver (ZFL) cells induced by microcystin-LR
IF 3.9
2区 农林科学
Q1 FISHERIES
引用次数: 0
Abstract
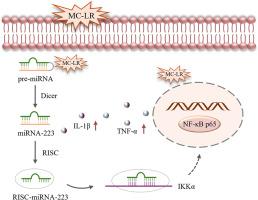
Microcystin-LR (MC-LR) is the most prevalent and toxic microcystin congeners. Various studies have provided clear evidence that MC-LR is capable of inducing hepatic inflammation; however, the mechanisms remain incompletely elucidated. In this study, the inflammatory reaction induced by MC-LR and the underlying mechanisms in zebrafish liver (ZFL) cells were investigated. The results demonstrated that MC-LR could penetrate ZFL cells and inhibit cellular activity. Moreover, MC-LR exposure elevated the level of inflammation-related markers, including IL-1β, TNF-α, IL-6, and IL-10, promoted nuclear translocation of NF-κB p65, and upregulated dre-miR-223 expression, suggesting that MC-LR may trigger an inflammatory reaction in ZFL cells via activation of the NF-κB pathway, with dre-miR-223 may participating in this regulatory process. Further functional experiments using synthetic dre-miR-223 mimics and inhibitors showed that overexpression of dre-miR-223 significantly elevated the expression levels of IL-1β and TNF-α, whereas inhibition of dre-miR-223 led to a marked decrease in these cytokines compared to those induced by MC-LR alone, indicating that dre-miR-223 enhances the MC-LR-induced inflammatory reaction through the positive regulation of pro-inflammatory cytokine production. Dual-luciferase reporter assays combined with functional analyses further revealed that dre-miR-223 directly targets IKKα, a key component of the NF-κB pathway, thereby modulating the inflammatory response. The findings of this study are crucial for understanding the inflammatory mechanisms triggered by MC-LR exposure in fish and for evaluating the associated health risks.
re- mir -223/IKKα/NF-κB促进微囊藻毒素lr诱导的斑马鱼肝脏(ZFL)细胞的炎症反应
微囊藻毒素- lr (MC-LR)是最普遍和毒性最强的微囊藻毒素同系物。各种研究已经提供了明确的证据表明MC-LR能够诱导肝脏炎症;然而,其机制仍未完全阐明。本研究探讨了MC-LR在斑马鱼肝脏(ZFL)细胞中诱导的炎症反应及其机制。结果表明,MC-LR能穿透ZFL细胞,抑制细胞活性。此外,MC-LR暴露提高炎症相关标志物,包括IL-1β、TNF-α、IL-6和IL-10的水平,促进NF-κB p65的核易位,上调re- mir -223的表达,表明MC-LR可能通过激活NF-κB途径引发ZFL细胞的炎症反应,而re- mir -223可能参与了这一调节过程。使用合成的re- mir -223模拟物和抑制剂进行的进一步功能实验表明,过表达re- mir -223可显著提高IL-1β和TNF-α的表达水平,而与单独使用MC-LR诱导的细胞因子相比,抑制re- mir -223可导致这些细胞因子的显著减少,这表明re- mir -223通过积极调节促炎细胞因子的产生来增强MC-LR诱导的炎症反应。双荧光素酶报告基因分析结合功能分析进一步揭示,dre-miR-223直接靶向NF-κB通路的关键组分IKKα,从而调节炎症反应。这项研究的发现对于理解MC-LR暴露在鱼类中引发的炎症机制和评估相关的健康风险至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fish & shellfish immunology
农林科学-海洋与淡水生物学
CiteScore
7.50
自引率
19.10%
发文量
750
审稿时长
68 days
期刊介绍:
Fish and Shellfish Immunology rapidly publishes high-quality, peer-refereed contributions in the expanding fields of fish and shellfish immunology. It presents studies on the basic mechanisms of both the specific and non-specific defense systems, the cells, tissues, and humoral factors involved, their dependence on environmental and intrinsic factors, response to pathogens, response to vaccination, and applied studies on the development of specific vaccines for use in the aquaculture industry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


