Applications of Conia-ene reaction in total synthesis of natural products
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The Conia-ene reaction is an intramolecular alkenylation (or alkylation) reaction occurring at the carbonyl α position of unsaturated ketones and serves as a highly efficient transformation for the construction of cyclic ketones, especially those within five- or six-membered rings frequently encountered within natural products. Compared with the traditional thermal-promoted transformation, Lewis acid catalyzed Conia-ene features mild conditions and widely tolerable functional groups thereby has been widely applied in the synthesis of natural products. In this review, we briefly introduce the development of the Conia-ene reaction, recent advancements in protocols, and a comprehensive review of its application in the total synthesis of natural products.
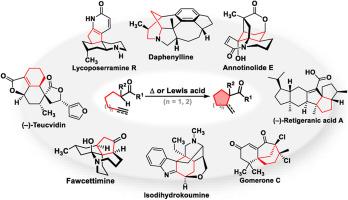
苯乙烯反应在天然产物全合成中的应用
Conia-ene反应是发生在不饱和酮的羰基α位置的分子内烯化(或烷基化)反应,是构建环酮的高效转化,特别是在天然产物中经常遇到的五或六元环内的环酮。与传统的热促进转化相比,Lewis酸催化的Conia-ene具有条件温和、官能团耐受性广的特点,因此在天然产物的合成中得到了广泛的应用。本文简要介绍了Conia-ene反应的发展、反应方案的最新进展,并对其在天然产物全合成中的应用进行了综述。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


