Coffee shell as a green reductant for iron recovery from pyrite cinder: synergy of biomass-driven reduction and acid leaching
IF 5
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
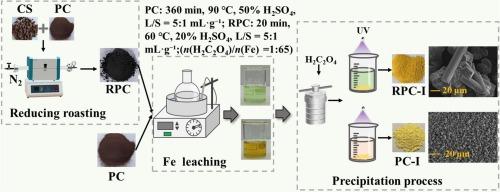
The inefficient recovery of iron from pyrite cinder (PC), a hazardous iron-rich byproduct of sulfuric acid production, remains a critical challenge due to chemical inertness of hematite and risks of toxic H2S generation. This study proposes a sustainable strategy integrating biomass reductive roasting with oxalic acid-assisted leaching to achieve high-efficiency iron recovery. Using coffee shell (CS) as a renewable reductant, PC was converted into reduced pyrite cinder (RPC) at 700 ℃ for 3 h (mass ratio PC:CS = 1:1), where hematite (Fe2O3) was stepwise reduced to reactive Fe3O4, FeO, and Fe(0), while simultaneously removing 35.78 % sulfur. The resultant RPC exhibited a porous structure with 21.4-fold increased surface area versus PC, facilitating rapid iron dissolution. Coupled with oxalic acid leaching (n(H2C2O4)/n(Fe) = 1:65), over 97 % iron extraction was achieved within 20 min under mild conditions (60 °C, 20 % H2SO4, L/S = 5:1 mL·g−1), outperforming untreated PC (<27 %) and yielding leachates with Fe(Ⅱ) concentrations up to 2.14 mol·L−1. Thermodynamic and kinetic analyses revealed that the process shifted from interfacial reaction control (PC) to diffusion-dominated mechanisms (RPC), driven by enhanced reducibility and microstructural modification. High-purity ferrous oxalate dihydrate was recovered at 85.6 % efficiency from RPC leachate via direct precipitation, avoiding the energy-intensive photoreduction required for Fe(Ⅲ)-rich PC leachate. This strategy achieved an overall iron recovery rate of 83.7 % from raw PC to battery-grade ferrous oxalate dihydrate, synchronizing hazardous tailings utilization, critical metal recovery, and agricultural waste upcycling while offering a sustainable blueprint for circular resource economies.
咖啡壳作为绿色还原剂从黄铁矿煤渣中回收铁:生物质驱动还原和酸浸的协同作用
硫铁矿渣(PC)是硫酸生产中富含铁的危险副产品,由于赤铁矿的化学惰性和产生有毒H2S的风险,从硫铁矿渣(PC)中回收铁的效率低下仍然是一个严峻的挑战。本研究提出了生物质还原焙烧与草酸辅助浸出相结合的可持续策略,以实现铁的高效回收。以咖啡壳(CS)为再生还原剂,在700℃下还原为还原性黄铁矿煤渣(RPC)(质量比PC:CS = 1:1) 3 h,其中赤铁矿(Fe2O3)逐步还原为活性Fe3O4、FeO和Fe(0),同时脱除35.78%的硫。制备的RPC具有多孔结构,比PC的表面积增加21.4倍,有利于铁的快速溶解。结合草酸浸出(n(H2C2O4)/n(Fe) = 1:65),在温和条件下(60°C, 20% H2SO4, L/S = 5:1 mL·g−1),在20分钟内获得97%以上的铁提取率,优于未处理的PC (< 27%),并产生铁(Ⅱ)浓度高达2.14 mol·L−1的浸出液。热力学和动力学分析表明,该过程由界面反应控制(PC)转变为扩散主导机制(RPC),由增强的还原性和微观结构修饰驱动。通过直接沉淀法从RPC渗滤液中回收高纯度草酸亚铁,效率为85.6%,避免了富含铁(Ⅲ)的PC渗滤液所需的高能量光还原。该战略实现了从PC原料到电池级二水合草酸亚铁的总体铁回收率为83.7%,实现了危险尾矿利用、关键金属回收和农业废弃物升级回收的同步,同时为循环资源经济提供了可持续的蓝图。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Minerals Engineering
工程技术-工程:化工
CiteScore
8.70
自引率
18.80%
发文量
519
审稿时长
81 days
期刊介绍:
The purpose of the journal is to provide for the rapid publication of topical papers featuring the latest developments in the allied fields of mineral processing and extractive metallurgy. Its wide ranging coverage of research and practical (operating) topics includes physical separation methods, such as comminution, flotation concentration and dewatering, chemical methods such as bio-, hydro-, and electro-metallurgy, analytical techniques, process control, simulation and instrumentation, and mineralogical aspects of processing. Environmental issues, particularly those pertaining to sustainable development, will also be strongly covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


