Reduced graphene oxide nanosheets supported platinum nanoparticles with enhanced mass activity for efficient hydrogen evolution
IF 5.1
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
Abstract
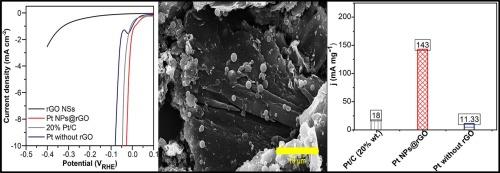
One strategy is synthesizing a low-loading platinum-based electrocatalyst and enhancing platinum utilization efficiency. In this study, platinum nanoparticles (Pt NPs) were embedded onto the reduced graphene oxide (rGO) nanosheet surface using solvothermal and chemical reduction processes. The experimental results illustrated that the developed Pt NPs/rGO NSs, which exhibit superior HER activity and excellent stability, require only a low Pt loading of 9.6 % wt. The fabricated Pt NPs@rGO demonstrated an overpotential of 27 mV, reaching a current density of 10 mA cm−2 with a lower Tafel slope of 34 mV dec−1 and high turnover frequency (0.25 s−1) in 0.5 M H2SO4. The HER performance of the Pt NPs@rGO electrocatalyst surpassed that of Pt without rGO (80 mV) and Pt/C (46 mV) electrodes. This enhancement may be due to the formation of Pt![]() O bonding on the rGO surface, leading to increased H atom adsorption. Furthermore, the strong electronic coupling between the Pt NPs and the rGO support is more favorable than that between Pt NPs and the carbon support (Pt/C, 20 % wt.). Notably, the mass activity of Pt NPs@rGO (143 mA mg−1) is 8-times greater than that of Pt/C (20 % wt.) (18 mA mg−1) at an overpotential of 27 mV, demonstrating that low-loading Pt NPs onto the rGO surface is more efficient than that of the benchmark Pt/C (20 % wt.). Impedance spectroscopy confirmed a strong electronic coupling between Pt NPs and rGO NSs, leading to high HER performance and fast electron mobility and charge transfer kinetics. The Pt NPs@rGO also exhibited excellent long-term stability over 24 h, indicating robust electronic interaction between Pt and rGO. The superior HER activity of the Pt NPs@rGO electrocatalyst is ascribed to the suitability of rGO as a support.
O bonding on the rGO surface, leading to increased H atom adsorption. Furthermore, the strong electronic coupling between the Pt NPs and the rGO support is more favorable than that between Pt NPs and the carbon support (Pt/C, 20 % wt.). Notably, the mass activity of Pt NPs@rGO (143 mA mg−1) is 8-times greater than that of Pt/C (20 % wt.) (18 mA mg−1) at an overpotential of 27 mV, demonstrating that low-loading Pt NPs onto the rGO surface is more efficient than that of the benchmark Pt/C (20 % wt.). Impedance spectroscopy confirmed a strong electronic coupling between Pt NPs and rGO NSs, leading to high HER performance and fast electron mobility and charge transfer kinetics. The Pt NPs@rGO also exhibited excellent long-term stability over 24 h, indicating robust electronic interaction between Pt and rGO. The superior HER activity of the Pt NPs@rGO electrocatalyst is ascribed to the suitability of rGO as a support.
还原氧化石墨烯纳米片支持的铂纳米颗粒具有增强的质量活性,用于有效的析氢
一种策略是合成低负荷铂基电催化剂,提高铂的利用效率。在这项研究中,铂纳米粒子(Pt NPs)通过溶剂热和化学还原过程嵌入到还原的氧化石墨烯(rGO)纳米片表面。实验结果表明,制备的Pt NPs/rGO NSs具有优异的HER活性和优异的稳定性,只需要9.6% wt的低Pt负载。制备的Pt NPs@rGO的过电位为27 mV,电流密度为10 mA cm−2,Tafel斜率较低,为34 mV dec−1,在0.5 M H2SO4中具有较高的转换频率(0.25 s−1)。Pt NPs@rGO电催化剂的HER性能优于不含rGO (80 mV)和Pt/C (46 mV)电极的Pt。这种增强可能是由于在还原氧化石墨烯表面形成PtO键,导致H原子吸附增加。此外,Pt NPs与还原氧化石墨烯载体之间的强电子耦合比Pt NPs与碳载体之间的强电子耦合更有利(Pt/C, 20% wt.)。值得注意的是,在过电位为27 mV时,Pt NPs@rGO (143 mA mg−1)的质量活性是Pt/C (20% wt.) (18 mA mg−1)的8倍,这表明低负荷Pt NPs在还原氧化石墨烯表面的效率高于基准Pt/C (20% wt.)。阻抗谱证实了Pt NPs和rGO NSs之间的强电子耦合,导致高HER性能和快速的电子迁移率和电荷转移动力学。Pt NPs@rGO在24小时内也表现出优异的长期稳定性,表明Pt和还原氧化石墨烯之间存在强大的电子相互作用。Pt NPs@rGO电催化剂优越的HER活性归因于氧化石墨烯作为载体的适宜性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


