Stable anode interphase enabled use of protic electrolytes in sodium metal batteries
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
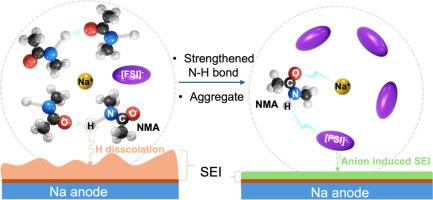
Sodium metal batteries based on liquid electrolytes are currently limited to using aprotic solvents, such as carbonate esters and ethers. This as protic solvents fundamentally have proton dissociation due to prevalent hydrogen bonding, leading to undesirable reactivity with the sodium metal anode. Our working hypothesis is that this reactivity can be controlled and reduced by replacing/disrupting the hydrogen bonding with other interactions. We present here the viability by using N-methyl-acetamide as an electrolyte solvent for sodium metal batteries, to which both Na+ cations and [FSI]- anions, from the NaFSI electrolyte salt used, can interact to modify the N![]() H bond strength. Combined with the formation of aggregates by careful composition control, the passivation of sodium metal anodes is effectively improved. Furthermore, distinctly different solid electrolyte interphases are formed, as compared to when using a conventional organic electrolyte, and excellent cycling stability of a full cell using Na3V2(PO4)3 as cathode is demonstrated, reaching an average Coulombic efficiency of 99.9 %. Overall, we show that protic solvents, given controlled proton activity, offer another route to possibly achieve practical sodium metal batteries.
H bond strength. Combined with the formation of aggregates by careful composition control, the passivation of sodium metal anodes is effectively improved. Furthermore, distinctly different solid electrolyte interphases are formed, as compared to when using a conventional organic electrolyte, and excellent cycling stability of a full cell using Na3V2(PO4)3 as cathode is demonstrated, reaching an average Coulombic efficiency of 99.9 %. Overall, we show that protic solvents, given controlled proton activity, offer another route to possibly achieve practical sodium metal batteries.
稳定的阳极间相使质子电解质在钠金属电池中的应用成为可能
基于液体电解质的钠金属电池目前仅限于使用非质子溶剂,如碳酸酯和醚。这是因为质子溶剂由于普遍存在的氢键而导致质子解离,导致与金属钠阳极的不良反应性。我们的工作假设是,这种反应性可以通过用其他相互作用取代/破坏氢键来控制和降低。我们在这里提出了用n -甲基乙酰胺作为钠金属电池的电解质溶剂的可行性,其中来自NaFSI电解质盐的Na+阳离子和[FSI]-阴离子可以相互作用来改变N-H键的强度。结合精心控制成分形成的聚集体,有效地改善了金属钠阳极的钝化。此外,与使用传统有机电解质相比,形成了明显不同的固体电解质界面,并且证明了以Na3V2(PO4)3为阴极的全电池具有优异的循环稳定性,平均库仑效率达到99.9%。总的来说,我们表明质子溶剂,在质子活性可控的情况下,提供了另一种可能实现实用钠金属电池的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


