Electrocatalytic upcycling of high-pressure captured CO2 to ethylene
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
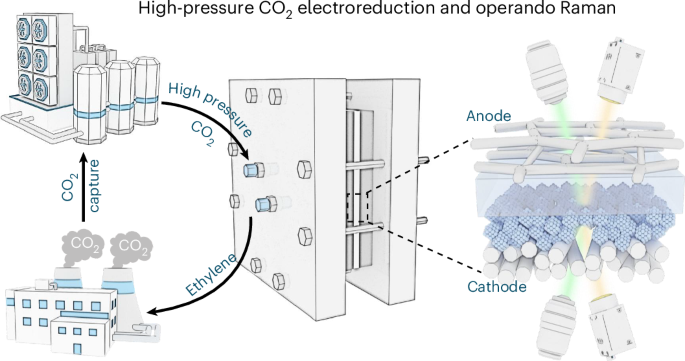
Electrochemical upcycling of captured CO2 under high pressure holds significant potential to bridge between CO2 emissions and hydrocarbon commodities, yet it remains underexplored. Here we convert gas-phase high-pressure captured CO2 into ethylene (C2H4) using a high-pressure membrane electrode assembly equipped with In/Cu catalysts, affording up to 85% Faradaic efficiency and 750 mA cm−2 partial current density under 20 bar. Theoretical calculations and operando studies link enhanced C–C coupling to the pressure-modulated *CO adsorption configuration and elevated CO2 coverage. High pressure also mitigates salt precipitation by relocating bicarbonate formation to the catalyst–membrane interface, enabling stable electrolysis for over 1,500 h at 600 mA cm−2. As a proof of concept, by recapturing residual CO2, the system delivers industrial-grade 99.9% purity C2H4, creating an opportunity to turn the otherwise costly CO2 capture into a profit. Energy analysis suggests that directly valorizing high-pressure captured CO2, instead of depressurizing and repressurizing, is essential to minimize energy consumption. Electrocatalytic CO2 conversion offers opportunities for producing sustainable fuels and chemicals, but achieving strong performance with realistic CO2 sources remains a challenge. Here a system is designed to use high-pressure captured CO2, and achieves 85% Faradaic efficiency and high-purity C2H4 for over 1,500 h.

高压捕集二氧化碳电催化升级回收制乙烯
在高压下对捕获的二氧化碳进行电化学升级回收,在二氧化碳排放和碳氢化合物产品之间具有巨大的桥梁潜力,但仍未得到充分开发。在这里,我们使用配备In/Cu催化剂的高压膜电极组件将气相高压捕获的CO2转化为乙烯(C2H4),在20 bar下提供高达85%的法拉第效率和750 mA cm - 2的分电流密度。理论计算和operando研究将增强的C-C耦合与压力调制的*CO吸附配置和提高的CO2覆盖率联系起来。高压还通过将碳酸氢盐重新定位到催化剂-膜界面来减轻盐的沉淀,从而在600毫安厘米−2下稳定电解超过1500小时。作为概念验证,通过重新捕获残留的二氧化碳,该系统提供了纯度为99.9%的工业级C2H4,从而创造了将原本昂贵的二氧化碳捕获转化为利润的机会。能源分析表明,直接对高压捕获的二氧化碳进行增压,而不是减压和再增压,对于最大限度地减少能源消耗至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


