Trophic transfer of CeO2 nanoparticles from clamworm to juvenile turbot and related changes in fish flesh quality
IF 17.6
引用次数: 0
Abstract
Engineered nanoparticles (ENPs) accumulate in marine sediments and exhibit adverse effects on benthic organisms. However, the effect of ENPs on marine benthic food chains is largely unknown. Herein, we investigated the trophic transfer and transformation of CeO2 ENPs within a simulated marine benthic food chain from clamworm (Perinereis aibuhitensis) to turbot (Scophthalmus maximus), as well as their effects on fish flesh quality. The results showed that Ce contents in turbot increased with the accumulation of CeO2 ENPs in clamworm, but no biomagnification of CeO2 ENPs occurred along this food chain. During trophic transfer, CeO2 ENPs in turbot experienced transformation from Ce(IV) to Ce(III). Importantly, CeO2 ENPs accumulated in the muscle of turbot and decreased the crude protein, total amino acid, and delicious amino acid contents, as well as the texture of the muscle. CeO2 ENPs induced the deterioration of flesh quality, which was mainly related to metabolism in muscle and intestinal disorders caused by oxidative stress. Specifically, CeO2 ENPs increased the relative abundance of Stenotrophomonas and Vibrio in the turbot intestine, while decreasing those of Lactobacillus, Bacillus, and Acinetobacter. Significant disturbances in purine and amino acid (aspartate, glutamate, glycine, etc.) metabolism in muscle were induced by CeO2 ENPs. Moreover, correlation analysis showed that microbiota dysbiosis was highly correlated with muscle metabolic dysfunction. Our study provides insights into the transfer and transformation of CeO2 ENPs and their interference with fish flesh quality via the gut–muscle axis, providing useful information on assessing ecological risk and food safety in marine environments.
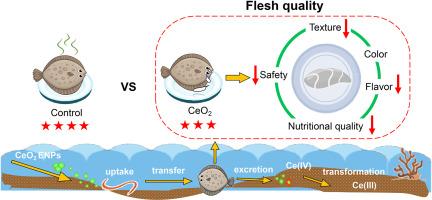
CeO2纳米颗粒从蛤蜊到大菱鲆幼鱼的营养转移及其对鱼肉品质的影响
工程纳米颗粒(ENPs)在海洋沉积物中积累,并对底栖生物产生不利影响。然而,ENPs对海洋底栖生物食物链的影响在很大程度上是未知的。在此,我们研究了CeO2 ENPs在模拟海洋底栖生物食物链中从蛤(Perinereis aibuhitensis)到大比目鱼(Scophthalmus maximus)的营养转移和转化,以及它们对鱼肉品质的影响。结果表明,随着CeO2 ENPs在蛤蜊体内的积累,大比目鱼中Ce含量增加,但CeO2 ENPs在该食物链中没有发生生物放大作用。在营养转移过程中,大菱鲆的CeO2 ENPs经历了从Ce(IV)到Ce(III)的转化。重要的是,CeO2 ENPs在大比目鱼肌肉中积累,降低了粗蛋白质、总氨基酸和美味氨基酸含量,降低了肌肉的质地。CeO2 ENPs诱导肉质恶化,主要与氧化应激引起的肌肉代谢紊乱和肠道紊乱有关。具体来说,CeO2 ENPs增加了大比目鱼肠道中窄养单胞菌和弧菌的相对丰度,而降低了乳杆菌、芽孢杆菌和不动杆菌的相对丰度。CeO2 ENPs显著干扰了肌肉中嘌呤和氨基酸(天冬氨酸、谷氨酸、甘氨酸等)的代谢。此外,相关分析显示,微生物群失调与肌肉代谢功能障碍高度相关。我们的研究揭示了CeO2 ENPs通过肠道-肌肉轴的转移和转化及其对鱼肉质量的干扰,为评估海洋环境中的生态风险和食品安全提供了有用的信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Eco-Environment & Health
环境科学与生态学-生态、环境与健康
CiteScore
11.00
自引率
0.00%
发文量
18
审稿时长
22 days
期刊介绍:
Eco-Environment & Health (EEH) is an international and multidisciplinary peer-reviewed journal designed for publications on the frontiers of the ecology, environment and health as well as their related disciplines. EEH focuses on the concept of “One Health” to promote green and sustainable development, dealing with the interactions among ecology, environment and health, and the underlying mechanisms and interventions. Our mission is to be one of the most important flagship journals in the field of environmental health.
Scopes
EEH covers a variety of research areas, including but not limited to ecology and biodiversity conservation, environmental behaviors and bioprocesses of emerging contaminants, human exposure and health effects, and evaluation, management and regulation of environmental risks. The key topics of EEH include:
1) Ecology and Biodiversity Conservation
Biodiversity
Ecological restoration
Ecological safety
Protected area
2) Environmental and Biological Fate of Emerging Contaminants
Environmental behaviors
Environmental processes
Environmental microbiology
3) Human Exposure and Health Effects
Environmental toxicology
Environmental epidemiology
Environmental health risk
Food safety
4) Evaluation, Management and Regulation of Environmental Risks
Chemical safety
Environmental policy
Health policy
Health economics
Environmental remediation
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


