Immunosuppressive spirocyclic labdane diterpenoids from Holocheila longipedunculata
IF 3.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Phytochemical study on Holocheila longipedunculata has revealed twenty previously undescribed spirocyclic labdane diterpenoids, named hololabdanes (1–20). Their chemical structures were elucidated through comprehensive spectroscopic analysis, and the absolute configuration of hololabdane A (1) was determined via single-crystal X-ray diffraction. Notably, hololabdane I (17) and 16-epi-hololabdane I (18) feature a rare pyrrolo [2,1-b] oxazolidinone moiety, while hololabdane J (19) and 2′-epi-hololabdane J (20) possess a distinctive peroxide bridge. Biological evaluations revealed that these spirocyclic labdanes exhibit potential immunosuppressive activity. In particular, compounds 5, 7, and 11 exhibited potent inhibition effects against IFN-γ secretion from T cells, with IC50 values of 1.21, 4.50, and 2.88 μM, respectively. Additionally, compounds 5 and 11 inhibited T cell proliferation, with IC50 values of 1.30 and 9.27 μM, respectively.
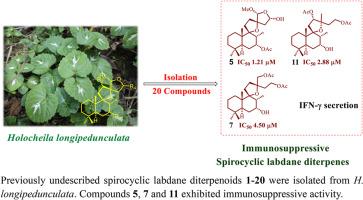
长柄海棠的免疫抑制螺环唇丹二萜
植物化学研究揭示了20种先前未被描述的螺环唇丹二萜,命名为hololabdanes(1-20)。通过综合光谱分析确定了它们的化学结构,并通过单晶x射线衍射确定了hololabdane A(1)的绝对构型。值得注意的是,hololabdane I(17)和16-epi-hololabdane I(18)具有罕见的吡咯[2,1-b]恶唑烷酮部分,而hololabdane J(19)和2 ' -epi-hololabdane J(20)具有独特的过氧化物桥。生物学评价显示,这些螺环内酯具有潜在的免疫抑制活性。其中化合物5、7和11对T细胞分泌IFN-γ具有明显的抑制作用,IC50值分别为1.21、4.50和2.88 μM。化合物5和11抑制T细胞增殖,IC50值分别为1.30 μM和9.27 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


