Influence of the synthesis route of lithium adsorbent Li/Al LDHs on physicochemical properties and extraction properties
IF 7.5
3区 工程技术
Q1 WATER RESOURCES
引用次数: 0
Abstract
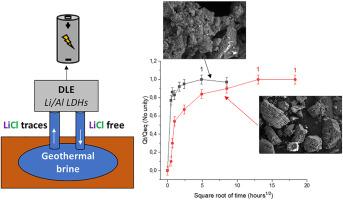
Lithium can be extracted from brines using a process commonly called Direct Lithium Extraction (DLE) using a lithium selective adsorbent as solid support. For that purpose, Li/Al layered double hydroxide (Li/Al LDH) are efficient materials for trapping Li+ in the vacant octahedral sites and chloride ions in their interlayer space. This article presents two distinct synthesis routes of Li/Al LDH materials, hydrothermal and coprecipitation, their characterizations and their Li extraction properties in synthetic brine. Indeed, the synthesis route impacts the physicochemical properties of the adsorbent and hence the Li+ extraction performances. A link between the structural and morphological properties of the adsorbents and the extraction properties was established. The material obtained by the coprecipitation method appears more effective for extracting lithium, as it is made up of grains of small particles with a greater pore volume and a larger exchange surface. This morphological property leads directly to a fast extraction kinetics, which is an essential point for a viable DLE process. This article also shows a close link between the LiCl content in the materials and the maximum achievable extraction capacity. The two active materials studied here have in fact the same maximum capacities due to an identical LiCl content in their crystalline structure, meaning that all sorption sites can be reached under optimal extraction conditions. It is therefore crucial to understand the influence of synthesis method on the extraction performance to optimize the process.
锂吸附剂Li/Al LDHs合成路线对其理化性质及萃取性能的影响
锂可以通过一种通常称为直接锂萃取(DLE)的方法从盐水中提取,这种方法使用锂选择性吸附剂作为固体载体。为此,Li/Al层状双氧根(Li/Al LDH)是捕获空八面体位置上Li+和层间空间氯离子的有效材料。本文介绍了水热法和共沉淀法两种不同的Li/Al LDH材料合成路线及其表征和在合成盐水中的Li萃取性能。事实上,合成路线会影响吸附剂的物理化学性质,从而影响Li+的提取性能。建立了吸附剂的结构和形态特性与萃取性能之间的联系。通过共沉淀法获得的材料更有效地提取锂,因为它是由小颗粒颗粒组成的,具有更大的孔隙体积和更大的交换面。这种形态特性直接导致了快速的萃取动力学,这是可行的DLE工艺的关键。本文还显示了材料中LiCl含量与最大可实现萃取能力之间的密切联系。由于晶体结构中LiCl含量相同,本文研究的两种活性材料实际上具有相同的最大容量,这意味着在最佳提取条件下可以达到所有吸附位点。因此,了解合成方法对提取性能的影响对优化工艺至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Resources and Industry
Social Sciences-Geography, Planning and Development
CiteScore
8.10
自引率
5.90%
发文量
23
审稿时长
75 days
期刊介绍:
Water Resources and Industry moves research to innovation by focusing on the role industry plays in the exploitation, management and treatment of water resources. Different industries use radically different water resources in their production processes, while they produce, treat and dispose a wide variety of wastewater qualities. Depending on the geographical location of the facilities, the impact on the local resources will vary, pre-empting the applicability of one single approach. The aims and scope of the journal include: -Industrial water footprint assessment - an evaluation of tools and methodologies -What constitutes good corporate governance and policy and how to evaluate water-related risk -What constitutes good stakeholder collaboration and engagement -New technologies enabling companies to better manage water resources -Integration of water and energy and of water treatment and production processes in industry
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


