Thermal-resistant semiconductive oligomers via bis(phenyl)fluorene-functionalized phenoxy-imine frameworks
IF 6.3
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
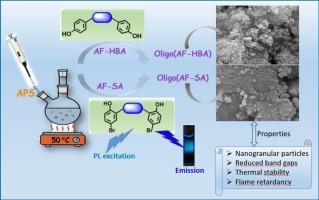
In this study, two oligo(phenoxy-imine)s containing a bis(phenyl)fluorene unit were synthesized. Initially, two Schiff base monomers (AF-HBA and AF-SA) were synthesized through the one–pot condensation reaction of 9,9-bis(4-aminophenyl)fluorene (AF) with 4-hydroxybenzaldehyde (HBA) and 5-bromosalicylaldehyde (SA), respectively. Subsequently, these Schiff base monomers were oligomerized to Oligo(AF-HBA) and Oligo(AF-SA) using ammonium persulphate (APS) as an oxidant. The structures of the synthesized compounds were confirmed through FT-IR, 1H NMR, 13C NMR, and UV–Vis spectroscopic analyses. Additional characterization techniques included optical measurements, electrochemical assessments, fluorescence (PL) and TG analyses. The AF-SA Schiff base exhibited blue photoluminescence in DMF, with a maximum emission intensity of 949 a.u. at the optimal excitation wavelength of 460 nm, and a quantum yield (QY) value of 18 % corresponding to the emission at 477 nm. The number-average molecular weights (Mw) and polydispersity indexes (PDI) of the oligomers were determined to be approximately 3750 Da with a PDI of 1.103 for Oligo(AF-HBA), while those for Oligo(AF-SA) were found to be 3900 Da with a PDI of 1.069 using GPC instrumentation. The low optical and electrochemical band gaps exhibited by the synthesized oligomers suggested their potential as semiconductive materials. The application of TGA and DSC analyses revealed that both Oligo(AF-HBA) and Oligo(AF-SA) exhibited thermal stability up to 234 °C and 294 °C respectively. Furthermore, their char amounts at 1000 °C were calculated to be relatively high at approximately 50.14 % for Oligo(AF-HBA) and 50.57 % for Oligo(AF-SA). These thermal properties, in conjunction with the obtained Limiting Oxygen Index (LOI) values, suggest that the synthesized oligomers hold potential for application in flame-retardant materials.
通过双(苯基)芴功能化苯氧亚胺框架的耐热半导体低聚物
本研究合成了两个含有双(苯基)芴单元的低聚(苯氧基亚胺)。首先,以9,9-双(4-氨基苯基)芴(AF)与4-羟基苯甲醛(HBA)和5-溴水杨醛(SA)为原料,通过一锅缩合反应分别合成了两个席夫碱单体AF-HBA和AF-SA。随后,这些希夫碱单体在过硫酸铵(APS)作为氧化剂的条件下被寡聚为Oligo(AF-HBA)和Oligo(AF-SA)。通过FT-IR、1H NMR、13C NMR和UV-Vis光谱分析证实了合成化合物的结构。其他表征技术包括光学测量,电化学评估,荧光(PL)和热重分析。AF-SA席夫碱在DMF中表现出蓝色光致发光,在460 nm的最佳激发波长下,最大发射强度为949 a.u.,对应于477 nm的发射量子产率(QY)值为18%。用GPC法测定了低聚物的数均分子量(Mw)和多分散指数(PDI)约为3750 Da,其中Oligo(AF-HBA)的PDI为1.103;Oligo(AF-SA)的数均分子量(Mw)为3900 Da, PDI为1.069。所合成的低聚物具有较低的光学和电化学带隙,表明它们具有作为半导体材料的潜力。TGA和DSC分析表明,Oligo(AF-HBA)和Oligo(AF-SA)的热稳定性分别达到234°C和294°C。此外,在1000°C时,它们的焦炭含量相对较高,Oligo(AF-HBA)约为50.14%,Oligo(AF-SA)约为50.57%。这些热性能,结合得到的极限氧指数(LOI)值,表明合成的低聚物在阻燃材料中具有应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


