Enantioselective Mannich reaction of 2-fluoro-1,3-diketones to ketimines: access to fluorinated α-amino acid derivatives
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
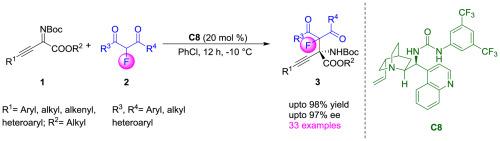
A highly enantioselective organocatalytic Mannich reaction between 2-fluoro-1,3-diketones and β,γ-alkynyl-α-imino esters has been developed, utilizing a chiral bifunctional urea catalyst. This strategy provides efficient access to a diverse range of fluorinated and non-fluorinated unnatural α-amino acid derivatives with excellent yields (up to 98 %) and enantioselectivities (up to 97 %). A wide array of substrates bearing diverse substituents is compatible with this reaction, showcasing its broad scope. The practicality of this approach was further highlighted by the successful scale-up reaction and subsequent transformations of the fluorinated amino acid derivatives.
2-氟-1,3-二酮与氯胺酮的对映选择性曼尼希反应:获得氟化α-氨基酸衍生物
利用手性双功能尿素催化剂,建立了2-氟-1,3-二酮与β,γ-炔基-α-亚胺酯的高对映选择性有机催化曼尼希反应。这一策略提供了各种氟化和非氟化的非天然α-氨基酸衍生物的有效途径,具有优异的收率(高达98%)和对映选择性(高达97%)。具有不同取代基的各种底物与该反应兼容,显示其广泛的范围。氟化氨基酸衍生物的成功放大反应和随后的转化进一步突出了这种方法的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


