All-orthogonal BINOLated BODIPY dimers: A synergistic strategy for advancing heavy-metal-free triplet photosensitizers
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
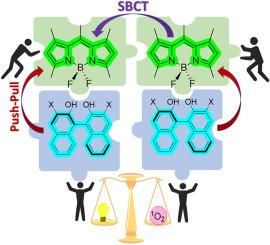
Covalently linked organic multichromophores are promising photoactive molecular scaffolds for developing valuable heavy-metal-free triplet photosensitizers. Among them, orthogonally connected BODIPY dimers and easily accessible at-boron BINOLated BODIPYs stand out owing to their efficient oxygen photosensitization, without the need for potentially toxic heavy atoms. In both approaches, the key photophysical mechanism enabling triplet state population involves a photoinduced intramolecular charge transfer by symmetry breaking in the orthogonally connected BODIPY dimers, or by electronic push-pull effect in the BINOL-BODIPY dyads. However, the potential synergistic effect of combining both strategies within a single molecular architecture remains unexplored. This work presents the first systematic study on the impact of integrating both photosensitizing approaches within a single BODIPY-based molecular framework. Our findings demonstrate that easy at-boron 3,3′-dibromoBINOLation serves as an effective chemical strategy to enhance triplet-based photosensitizing performance, without relying on potentially toxic heavy atoms such as transition metals. These results are expected to lay the foundations for the rational design of next-generation of low-cost BODIPY-based triplet photosensitizers for applications beyond heavy-metal-free photodynamic therapy, such as photocatalysis.
全正交双酚化BODIPY二聚体:推进无重金属三重态光敏剂的协同策略
共价连接的有机多发色团是开发有价值的无重金属三重态光敏剂的有前途的光活性分子支架。其中,正交连接的BODIPY二聚体和易于获得的在硼BINOLated BODIPY因其高效的氧光敏性而不需要潜在的有毒重原子而脱颖而出。在这两种方法中,实现三重态居群的关键光物理机制包括通过正交连接的BODIPY二聚体中的对称破断或BINOL-BODIPY二聚体中的电子推拉效应引起的光诱导分子内电荷转移。然而,在单一分子结构中结合这两种策略的潜在协同效应仍未被探索。这项工作提出了在一个单一的基于bodip的分子框架内整合两种光敏方法的影响的第一个系统研究。我们的研究结果表明,简单的-硼3,3 ' -二溴化是一种有效的化学策略,可以增强三重基光敏性能,而不依赖于潜在的有毒重原子,如过渡金属。这些结果有望为合理设计下一代低成本的基于bodipy的三重态光敏剂奠定基础,用于光催化等无重金属光动力治疗以外的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


