Tumor transcriptome-wide expression classifiers predict treatment sensitivity in advanced prostate cancers
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Advanced prostate cancers respond to hormone therapy but outcomes vary and no predictive tests exist for informed treatment selection. To identify novel biomarker-treatment pairings, we examined associations between biological pathways and 14-year survival outcomes of patients randomized in practice-changing phase 3 trials (testing docetaxel or abiraterone). We included transcriptome-wide expression signatures and immunohistochemistry markers (Ki-67 and PTEN) on prostate tumors from 1,523 patients (832 metastatic). Tumor androgen receptor signaling is associated with longer survival, whereas increased proliferation predicted shorter survival. In a pre-specified analysis, the previously identified decipher RNA signature was both prognostic and predicted survival benefit from docetaxel for metastatic cancers (biomarker-docetaxel interaction p = 0.039). Additionally, transcriptome-based classification of PTEN inactivation identified tumors more likely to have PTEN protein loss (p = 4 × 10−37) and metabolically perturbed metastatic cancers that had shorter survival with hormone therapies (p < 0.001) but exhibited docetaxel sensitivity (biomarker-docetaxel interaction p = 0.002). Transcriptome classifiers predict docetaxel benefit and could be clinically implemented for improved patient management.
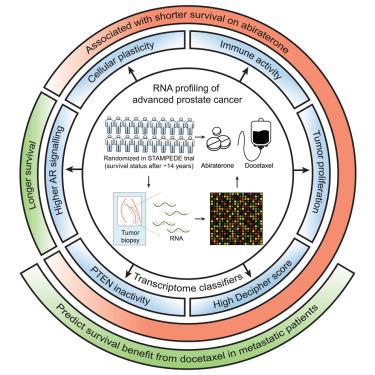
肿瘤转录组全表达分类器预测晚期前列腺癌的治疗敏感性
晚期前列腺癌对激素治疗有反应,但结果各不相同,没有预测治疗选择的试验。为了确定新的生物标志物-治疗配对,我们研究了在改变实践的3期试验(测试多西他赛或阿比特龙)中随机分配的患者的生物学途径与14年生存结果之间的关系。我们纳入了1523例(832例转移性)前列腺肿瘤的转录组表达特征和免疫组织化学标记(Ki-67和PTEN)。肿瘤雄激素受体信号与较长的生存期相关,而增殖增加则预示较短的生存期。在一项预先指定的分析中,先前鉴定的破译RNA标记既可以预测多西紫杉醇治疗转移性癌症的预后,也可以预测多西紫杉醇的生存益处(生物标志物-多西紫杉醇相互作用p = 0.039)。此外,基于转录组的PTEN失活分类鉴定出更可能发生PTEN蛋白丢失的肿瘤(p = 4 × 10−37)和代谢干扰的转移性癌症,这些肿瘤在激素治疗下生存时间较短(p < 0.001),但表现出多西他赛敏感性(生物标志物-多西他赛相互作用p = 0.002)。转录组分类器可预测多西他赛的疗效,并可用于临床改善患者管理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


