Long-range deployment of tumor-antigen-specific cytotoxic T lymphocytes inhibits lung metastasis of breast cancer
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Tumor-antigen-specific CD8+ T cells (CTLs) are the main effector immunocytes in anti-tumor immunity, but their systemic deployment against cancer metastasis remains uncharacterized. Here, we found that the abundance of tumor-specific CD103+CD8+ T cells in the tumor-draining lymph nodes (TDLNs) was associated with improved lung-metastasis-free survival in breast cancer patients. In mouse cancer models, CD103+CD8+ T cells were primed in TDLNs and recruited to the lungs via C-C motif chemokine ligand 5/receptor 9 (CCL25/CCR9) signaling to inhibit metastasis through antigen-specific immunity. Furthermore, extracellular vesicles (EVs) from early- and late-stage tumors differentially polarized alveolar macrophages to release CCL25 and IDO1, respectively, and the latter impaired pulmonary CD103+CD8+ T cell deployment, facilitating lung metastasis. Depleting IDO1 effectively rescued CD103+CD8+ T cell-mediated protection against lung metastasis. These findings exemplified long-range deployment of adaptive immunity to protect distant organs from metastasis, highlighting the therapeutic potential of reconstituting effector immune cell deployment (EICD) for cancer treatment.
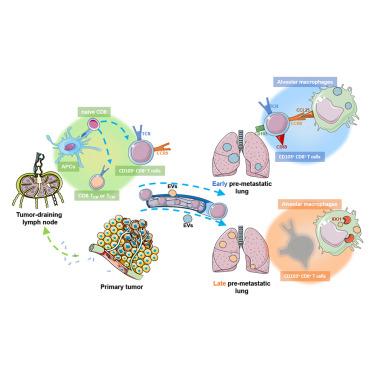
肿瘤抗原特异性细胞毒性T淋巴细胞的远程部署抑制乳腺癌的肺转移
肿瘤抗原特异性CD8+ T细胞(ctl)是抗肿瘤免疫的主要效应免疫细胞,但其在肿瘤转移中的全身性部署尚不清楚。在这里,我们发现肿瘤引流淋巴结(tdln)中肿瘤特异性CD103+CD8+ T细胞的丰度与乳腺癌患者肺无转移生存率的提高有关。在小鼠肿瘤模型中,CD103+CD8+ T细胞在tdln中启动,并通过C-C基序趋化因子配体5/受体9 (CCL25/CCR9)信号传导募集到肺部,通过抗原特异性免疫抑制转移。此外,来自早期和晚期肿瘤的细胞外囊泡(EVs)分别分化肺泡巨噬细胞以释放CCL25和IDO1,后者破坏肺部CD103+CD8+ T细胞的部署,促进肺转移。耗尽IDO1有效地挽救了CD103+CD8+ T细胞介导的肺转移保护。这些发现证明了适应性免疫的远程部署以保护远处器官免受转移,强调了重建效应免疫细胞部署(EICD)用于癌症治疗的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


