A split-site E3 ligase mechanism enables ZNFX1 to ubiquitinate and cluster single-stranded RNA into ubiquitin-coated nucleoprotein particles
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Eukaryotic cells use a multi-layered immune response to combat intracellular pathogens. The ubiquitin ligase ZNFX1 has emerged as a crucial yet little understood player that regulates the immune response while protecting against RNA viruses. Our study unveils the molecular mechanism of ZNFX1, mediated by the joint activity of a helicase serving as a nucleic acid sensor and a non-conventional E3 module featuring a split active site. We demonstrate that single-stranded RNA stimulates E3 activity by fostering dimerization of ZNFX1 subunits that translocate along nucleic acid tracks. Juxtaposed E3 domains complement each other, leading to the ubiquitination of ZNFX1 itself and engaged RNA molecules, while clustering nucleic acids into dense nucleoprotein particles. We show that the E3 ligase activity of ZNFX1 protects cells during an immune response and propose that ubiquitin-coated particles formed by ZNFX1 represent part of an ancient mechanism to regulate both foreign and host RNA in the cell.
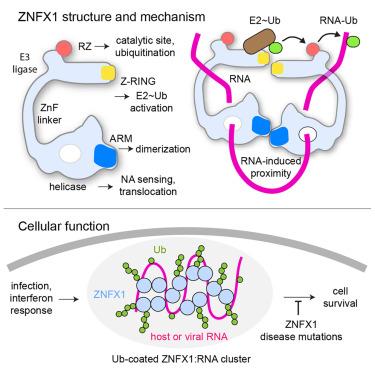
分裂位点E3连接酶机制使ZNFX1能够泛素化并将单链RNA聚集成泛素包被的核蛋白颗粒
真核细胞使用多层免疫反应来对抗细胞内病原体。泛素连接酶ZNFX1已经成为一个至关重要但鲜为人知的角色,它在调节免疫反应的同时保护机体免受RNA病毒的侵害。我们的研究揭示了ZNFX1的分子机制,它是由作为核酸传感器的解旋酶和具有分裂活性位点的非常规E3模块的联合活性介导的。我们证明单链RNA通过促进沿核酸轨迹易位的ZNFX1亚基的二聚化来刺激E3活性。并列的E3结构域相互补充,导致ZNFX1自身泛素化,并参与RNA分子,同时将核酸聚集成致密的核蛋白颗粒。我们发现ZNFX1的E3连接酶活性在免疫应答中保护细胞,并提出由ZNFX1形成的泛素包被颗粒代表了调节细胞中外源和宿主RNA的古老机制的一部分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


