Enhanced hydrogen production performance and stability simultaneously in aqueous-phase reforming of methanol over Pt/V2O3-V8C7 based on strong-weak metal support interactions
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
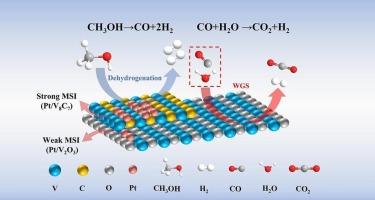
Aqueous phase reforming of methanol (APRM) is an effective method for the storage and transportation of hydrogen (H2). However, this approach faces challenges due to the limited activity and stability of metal-supported catalysts. In this study, Pt/V2O3-V8C7 metal-supported catalysts were prepared for APRM and the H2 production of Pt/V2O3-V8C7 at 200 °C was 96.52 mmol·g−1·h−1, which is 1.83 times and 1.71 times higher than the H2 production performance of Pt/V2O3 and Pt/V8C7, respectively. Moreover, the catalyst demonstrated remarkable stability after 10 in situ cycling tests. Structural characterization revealed that there is a strong metal-support interaction (SMSI) between Pt and V8C7, and a weak metal-support interaction (WMSI) between Pt and V2O3, which inhibits the sintering of Pt on the metal-supported catalyst. Furthermore, a straightforward experimental method has been developed that enables accurate inference of the catalytic reaction mechanism in APRM without the need for in situ material characterization. The reaction mechanism indicates that the Pt-V8C7 interface serves as the active site for the methanol decomposition step, while the surface of V2O3 acts as the active site for the water–gas shift reaction step. This strategy of strong–weak MSI offers a novel approach to enhancing both the stability and H2 production performance in APRM.

基于强-弱金属载体相互作用的Pt/V2O3-V8C7上甲醇水相重整同时增强制氢性能和稳定性
甲醇水相重整(APRM)是一种有效的储存和运输氢气(H2)的方法。然而,由于金属负载催化剂的活性和稳定性有限,这种方法面临挑战。本研究制备了Pt/V2O3-V8C7金属负载型APRM催化剂,在200 ℃下,Pt/V2O3-V8C7的产氢性能为96.52 mmol·g−1·h−1,分别是Pt/V2O3和Pt/V8C7的1.83倍和1.71倍。此外,经过10次原位循环试验,该催化剂表现出显著的稳定性。结构表征表明,Pt与V8C7之间存在强的金属-载体相互作用(SMSI),而Pt与V2O3之间存在弱的金属-载体相互作用(WMSI),抑制了Pt在金属负载催化剂上的烧结。此外,已经开发了一种简单的实验方法,可以在不需要原位材料表征的情况下准确推断APRM中的催化反应机理。反应机理表明,Pt-V8C7界面是甲醇分解步骤的活性位点,而V2O3表面是水气转换反应步骤的活性位点。这种强-弱MSI策略为提高APRM的稳定性和产氢性能提供了一种新的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


