High-throughput microfluidic generation of self-assembled, uniform 3D vascularized and mineralized bone organoids without matrix biomaterials
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
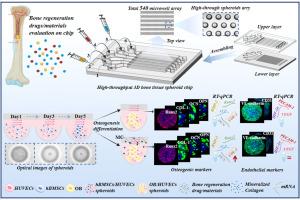
Bone organoids hold great promise for modeling bone-related diseases, improving bone injury repair strategies, and enabling high-throughput drug screening. However, conventional approaches rely heavily on matrix biomaterials—such as Matrigel, collagen gels, or 3D-printed scaffolds—which introduce undefined parameters, pose manufacturing complexities, and raise cost barriers, potentially limiting clinical translation. To address these challenges, we present a novel high-throughput microfluidic chip that generates 540 uniformly sized, scaffold-free 3D bone organoids simultaneously within six independent channels. By co-cultivating human bone marrow mesenchymal stem cells (hBMSCs), human umbilical vein endothelial cells (HUVECs), and mineralized collagen (MC), self-assembled bone organoids formed by the third day and progressively compacted over time, exhibiting enhanced cell viability and proliferation. The inclusion of MC upregulated multiple osteogenic markers (OCN, ALP, COL-1, RUNX2, and BMP-2), while endothelial markers (PECAM-1, HIF-1α, and VEGF) remained consistently expressed, reflecting stable vascularization and mineralization potential. Overall, this high-throughput, matrix-free microfluidic platform offers a biomimetic environment for the investigation of osteogenesis and angiogenesis and holds significant promise for advanced material assessment, drug screening, and disease modeling.
无基质生物材料的自组装、均匀三维血管化和矿化骨类器官的高通量微流控生成
骨类器官在骨相关疾病建模、改善骨损伤修复策略和实现高通量药物筛选方面具有很大的前景。然而,传统的方法严重依赖于基质生物材料,如Matrigel、胶原蛋白凝胶或3d打印支架,这些材料引入了未定义的参数,造成了制造的复杂性,并提高了成本壁垒,潜在地限制了临床转化。为了解决这些挑战,我们提出了一种新型的高通量微流控芯片,它可以在六个独立的通道内同时生成540个均匀大小的无支架3D骨类器官。通过共同培养人骨髓间充质干细胞(hBMSCs)、人脐静脉内皮细胞(HUVECs)和矿化胶原(MC),自组装的类骨器官在第三天形成,并随着时间的推移逐渐压缩,显示出增强的细胞活力和增殖能力。MC的加入上调了多种成骨标志物(OCN、ALP、COL-1、RUNX2和BMP-2),而内皮标志物(PECAM-1、HIF-1α和VEGF)的表达保持一致,反映了稳定的血管化和矿化潜力。总的来说,这种高通量、无基质的微流控平台为研究成骨和血管生成提供了一个仿生环境,并在先进的材料评估、药物筛选和疾病建模方面具有重要的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


