The use of highly polar additives to reduce the degradation of Cu/ZnO/Al2O3 catalysts during the gas phase hydrogenation of CO2 to methanol and CO
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The impact of catalyst deactivation due to sintering on the activity and properties of the Cu/ZnO/Al2O3 system in CO2 hydrogenation to methanol was systematically investigated. Additionally, the effects of various additives on methanol yield and catalyst stability during CO2/H2 hydrogenation were evaluated. Additives such as methanol were confirmed to enhance catalytic activity, while components with high dipole moments exhibited a poisoning effect. Interestingly, despite their deactivating influence on activity, these polar additives significantly improved catalyst stability. It is presumed that highly polar species adsorb onto the catalyst surface, displacing water that would otherwise promote crystal growth of copper and zinc oxides, leading to deactivation. A combined additive strategy using methanol (to boost activity) and propylene carbonate (to enhance stability) offers a novel approach to significantly slow down Cu/ZnO/Al2O3 catalyst deactivation during gas-phase CO2 hydrogenation, without compromising initial activity compared to conventional additive-free systems. Thus, catalyst stability can be effectively improved through the addition of appropriate components to the feed, without sacrificing reaction rate.
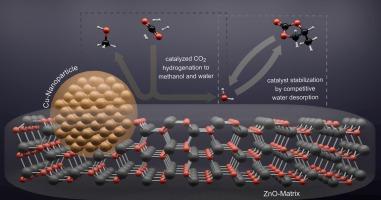
采用高极性添加剂减少Cu/ZnO/Al2O3催化剂在CO2气相加氢制甲醇和CO过程中的降解
系统研究了烧结失活对Cu/ZnO/Al2O3体系CO2加氢制甲醇活性和性能的影响。此外,还考察了不同添加剂对CO2/H2加氢过程中甲醇收率和催化剂稳定性的影响。甲醇等添加剂可以增强催化活性,而高偶极矩组分则表现出中毒效应。有趣的是,尽管这些极性添加剂对活性有失活影响,但它们显著提高了催化剂的稳定性。据推测,高极性物质吸附在催化剂表面,取代了水,否则会促进铜和锌氧化物的晶体生长,导致失活。使用甲醇(提高活性)和碳酸丙烯酯(提高稳定性)的组合添加剂策略提供了一种新方法,可以显着减缓Cu/ZnO/Al2O3催化剂在气相CO2加氢过程中的失活,而与传统的无添加剂系统相比,不会影响初始活性。因此,在不牺牲反应速率的前提下,通过在进料中添加适当的组分,可以有效地提高催化剂的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


