Fall-like acclimation alters mitochondrial function and substrate use in freeze-tolerant crickets
IF 2.9
2区 生物学
Q2 BIOLOGY
引用次数: 0
Abstract
Acclimation to low temperatures in insects involves complex metabolic adjustments to ensure survival. Notably, cryoprotectants including glycerol, trehalose and proline can accumulate in insects to promote freeze tolerance. Interestingly, these metabolites can also be converted to metabolic fuels sustaining mitochondrial ATP production. This study investigates the metabolic shifts in the freeze-tolerant cricket Gryllus veletis during fall-like acclimation, focusing on mitochondrial function and its possible link with cryoprotectant accumulation. We hypothesized that fall-like acclimation promotes a shift in mitochondrial substrate utilization within the fat body tissue of crickets, transitioning from a reliance on substrates that donate electrons to complex I (e.g., carbohydrates and fatty acids) toward increased utilization of alternative substrates, such as glycerol-3-phosphate (G3P), which can be derived from glycerol. We demonstrated that acclimation leads to lower glycogen content in the fat body, concomitant with increased pyruvate kinase activity, suggesting increased carbon flux through glycolysis. Moreover, we observed decreased overall mitochondrial oxygen consumption and enzymatic activity of cytochrome c oxidase. Interestingly, while overall mitochondrial capacity decreased, the relative contribution of G3P to mitochondrial metabolism increased, supported by increased enzymatic activity of mitochondrial G3P dehydrogenase. Thus, fall-like acclimation appears to induce a metabolic shift in crickets, redirecting metabolites away from the tricarboxylic acid cycle and towards the production of G3P, which subsequently sustains mitochondrial respiration. This study reveals a key mechanism associated with the development of freeze tolerance in crickets, which could also be important for other cold- and freeze-tolerant insect species.
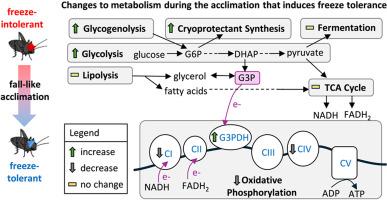
在抗冻蟋蟀中,类似秋天的驯化改变了线粒体功能和底物的使用
昆虫对低温的适应涉及复杂的代谢调节,以确保生存。值得注意的是,包括甘油、海藻糖和脯氨酸在内的冷冻保护剂可以在昆虫体内积累,以提高抗冻性。有趣的是,这些代谢物也可以转化为维持线粒体ATP生产的代谢燃料。本研究研究了耐冻蟋蟀在秋季样驯化过程中的代谢变化,重点研究了线粒体功能及其与冷冻保护剂积累的可能联系。我们假设,类似瀑布的驯化促进了蟋蟀脂肪体组织中线粒体底物利用的转变,从依赖向复合体I提供电子的底物(例如碳水化合物和脂肪酸)过渡到增加对替代底物的利用,例如甘油-3-磷酸(G3P),它可以从甘油中提取。我们证明,驯化导致脂肪体中糖原含量降低,同时丙酮酸激酶活性增加,表明糖酵解增加了碳通量。此外,我们观察到线粒体总体耗氧量和细胞色素c氧化酶的酶活性下降。有趣的是,虽然线粒体总体容量下降,但G3P对线粒体代谢的相对贡献增加,这得到了线粒体G3P脱氢酶活性增加的支持。因此,类似于坠落的适应似乎诱导了蟋蟀的代谢转变,将代谢物从三羧酸循环转向G3P的产生,G3P随后维持线粒体呼吸。这项研究揭示了蟋蟀耐寒性发展的关键机制,这对其他耐寒和耐冻昆虫物种也很重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of thermal biology
生物-动物学
CiteScore
5.30
自引率
7.40%
发文量
196
审稿时长
14.5 weeks
期刊介绍:
The Journal of Thermal Biology publishes articles that advance our knowledge on the ways and mechanisms through which temperature affects man and animals. This includes studies of their responses to these effects and on the ecological consequences. Directly relevant to this theme are:
• The mechanisms of thermal limitation, heat and cold injury, and the resistance of organisms to extremes of temperature
• The mechanisms involved in acclimation, acclimatization and evolutionary adaptation to temperature
• Mechanisms underlying the patterns of hibernation, torpor, dormancy, aestivation and diapause
• Effects of temperature on reproduction and development, growth, ageing and life-span
• Studies on modelling heat transfer between organisms and their environment
• The contributions of temperature to effects of climate change on animal species and man
• Studies of conservation biology and physiology related to temperature
• Behavioural and physiological regulation of body temperature including its pathophysiology and fever
• Medical applications of hypo- and hyperthermia
Article types:
• Original articles
• Review articles
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


