Sustainable recovery of nickel and aluminum from spent NiAl2O4 catalysts via soda roasting-water leaching: Disruption of NiAl2O4 and leaching kinetic
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
The widespread use of NiO/Al2O3 catalysts in the petrochemical industry generates hazardous spent materials due to the formation of refractory nickel-aluminum spinel (NiAl2O4), which complicates metal recovery and exacerbates resource inefficiency. Traditional direct acid and alkali leaching cannot recover Ni and Al due to excellent stable structure of NiAl2O4. Here, we propose a simple soda roasting-water leaching strategy to disrupt the spinel lattice and recover Ni and Al with high efficiency. By roasting spent catalysts with Na2CO3 at 1000 °C, Na + ions substituted Al3+ in the octahedral sites, which destroyed the spinel framework and converted Al2O3/NiAl2O4 into water-soluble NaAlO2. Over 98.31 % of Al was selectively leached at 90 °C, followed by pH-controlled precipitation and calcination at 600 °C to regenerate γ-Al2O3 with the purity of 98.21 %. Residual NiO was recovered via 2 M H2SO4 leaching, with Fe impurities removed through pH-selective precipitation. The kinetic analysis revealed a chemically controlled leaching mechanism (Ea = 47.61 kJ/mol). Final Ni(OH)2 through calcination at 400 °C yielded reusable NiO. This approach achieved >98 % metal recovery, minimized waste, and aligned with circular economy principles by transforming hazardous spent catalysts into high-purity industrial feedstocks.
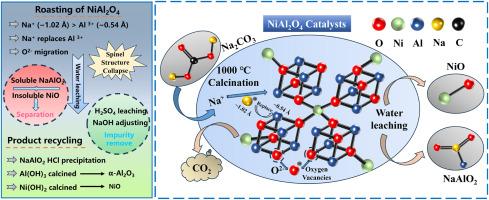
碱焙烧-水浸法从废NiAl2O4催化剂中可持续回收镍和铝:NiAl2O4的破坏和浸出动力学
由于镍铝尖晶石(NiAl2O4)的难降解,在石油化工行业中广泛使用NiO/Al2O3催化剂,产生了危险的废材料,使金属回收复杂化,加剧了资源的低效率。由于NiAl2O4具有优异的稳定结构,传统的直接酸碱浸出无法回收Ni和Al。在此,我们提出了一种简单的苏打焙烧-水浸策略,以破坏尖晶石晶格并高效回收Ni和Al。用Na2CO3在1000℃下焙烧废催化剂,Na +离子取代了八面体上的Al3+,破坏了尖晶石骨架,使Al2O3/NiAl2O4转化为水溶性NaAlO2。在90℃条件下选择性浸出98.31%以上的Al,然后在600℃条件下进行ph控制沉淀和煅烧,再生纯度为98.21%的γ-Al2O3。通过2 M H2SO4浸出回收残余NiO,通过ph选择性沉淀去除Fe杂质。动力学分析揭示了化学控制浸出机制(Ea = 47.61 kJ/mol)。最终的Ni(OH)2在400°C下煅烧得到可重复使用的NiO。这种方法实现了98%的金属回收率,最大限度地减少了浪费,并通过将危险的废催化剂转化为高纯度的工业原料,符合循环经济原则。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


