Twist1 promoting neointima hyperplasia after vascular injury in diabetes via regulating hyperglycemia-induced phenotype switching of vascular smooth muscle cells
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background and aims
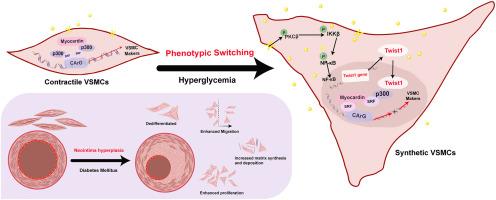
Neointimal hyperplasia is a key pathology in Type 2 Diabetes Mellitus (T2DM) vascular complications. It involves phenotypic switching of vascular smooth muscle cells (VSMCs) triggered by hyperglycemia, though the exact mechanisms remain unclear.
Methods
We employed Twist1 vascular smooth muscle-specific knockout mice with carotid artery ligation in a T2DM model to study Twist1's role in diabetic neointimal hyperplasia. In vitro, we examined how hyperglycemia via Pkcβ/Ikkβ/Nf-κb pathway affects Twist1's regulation of contractile proteins, matrix molecules, cell morphology, migration, and proliferation in rat VSMCs using Western blotting, immunofluorescence, wound healing assays, and EdU incorporation. Co-immunoprecipitation and colocalization assessed how Twist1-p300 interaction under high glucose affects Myocardin-Srf binding.
Results
Twist1 was significantly upregulated in VSMCs of T2DM mice. Vascular smooth muscle-specific Twist1 knockout reduced neointimal formation after vascular injury in T2DM. High glucose activated Pkcβ/Ikkβ/Nf-κb pathway, promoting Twist1 upregulation and nuclear translocation, decreasing contractile protein expression while increasing matrix molecules and VSMC proliferation/migration. Mechanistically, upregulated Twist1 increased p300 binding, blocking p300's transcriptional co-activation of Myocardin/Srf and inhibiting contractile gene transcription in VSMCs.
Conclusions
Hyperglycemia activates the PKCβ/IKKβ/NF-κB pathway, upregulating Twist1 and promoting its nuclear translocation. Twist1 binding to p300 inhibits Myocardin/SRF-mediated contractile gene transcription, leading to VSMC phenotypic switching and neointimal hyperplasia. These findings highlight Twist1 as a potential therapeutic target for diabetic vascular complications.
Twist1通过调节高血糖诱导的血管平滑肌细胞表型转换促进糖尿病血管损伤后的新生内膜增生
背景与目的内膜增生是2型糖尿病(T2DM)血管并发症的关键病理。它涉及由高血糖引发的血管平滑肌细胞(VSMCs)的表型转换,尽管确切的机制尚不清楚。方法采用颈动脉结扎T2DM模型小鼠Twist1血管平滑肌特异性敲除,研究Twist1在糖尿病新内膜增生中的作用。在体外,我们通过Western blotting、免疫荧光、创面愈合试验和EdU结合研究了Pkcβ/Ikkβ/Nf-κb途径的高血糖如何影响Twist1对大鼠VSMCs中收缩蛋白、基质分子、细胞形态、迁移和增殖的调节。共免疫沉淀和共定位评估了高糖条件下Twist1-p300相互作用如何影响心肌素- srf结合。结果T2DM小鼠VSMCs中stwist1表达明显上调。血管平滑肌特异性Twist1敲除可减少T2DM血管损伤后新内膜的形成。高糖激活Pkcβ/Ikkβ/Nf-κb通路,促进Twist1上调和核易位,降低收缩蛋白表达,增加基质分子和VSMC增殖/迁移。在机制上,上调的Twist1增加了p300的结合,阻断了p300对心肌素/Srf的转录共激活,抑制了VSMCs中收缩基因的转录。结论低血糖可激活PKCβ/IKKβ/NF-κB通路,上调Twist1,促进其核易位。Twist1结合p300抑制了心肌素/ srf介导的收缩基因转录,导致VSMC表型转换和新生内膜增生。这些发现突出了Twist1作为糖尿病血管并发症的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis
医学-外周血管病
CiteScore
9.80
自引率
3.80%
发文量
1269
审稿时长
36 days
期刊介绍:
Atherosclerosis has an open access mirror journal Atherosclerosis: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atherosclerosis brings together, from all sources, papers concerned with investigation on atherosclerosis, its risk factors and clinical manifestations. Atherosclerosis covers basic and translational, clinical and population research approaches to arterial and vascular biology and disease, as well as their risk factors including: disturbances of lipid and lipoprotein metabolism, diabetes and hypertension, thrombosis, and inflammation. The Editors are interested in original or review papers dealing with the pathogenesis, environmental, genetic and epigenetic basis, diagnosis or treatment of atherosclerosis and related diseases as well as their risk factors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


