Synthesis and characterization of octa(aminophenyl) silsesquioxane through amination of octa(nitrophenyl) silsesquioxane using B2(OH)4 at room temperature
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Octa(aminophenyl) silsesquioxane (OAPS), a promising polyhedral oligomeric silsesquioxane (POSS) derivative, exhibits exceptional structural and functional properties, making it highly suitable for advanced material applications. In this study, we developed an efficient and sustainable synthesis method for OAPS, utilizing tetrahydroxydiboron [B2(OH)4] as a reducing agent and 4,4′-bipyridine as a catalytic mediator. This strategy streamlines the synthetic procedure through a metal-free approach, operating under mild conditions while achieving high yields and demonstrating exceptional process stability. Initially, octa(nitrophenyl) silsesquioxane (ONPS) was synthesized as the precursor. Through a comprehensive investigation and evaluation of different reducing agents, catalysts, and their quantities, a novel reduction strategy was developed, enabling the complete and efficient conversion of nitro groups to amino groups. The obtained OAPS was characterized by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR), while matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) confirmed its high purity and structural integrity through molecular weight analysis. A proposed mechanistic pathway for the reduction process, facilitated by the synergistic action of B2(OH)4 and 4,4′-bipyridine, is also proposed. The synthesized OAPS demonstrated remarkable thermal stability, with an initial decomposition temperature of 430.2 °C under nitrogen and 399.3 °C under air, highlighting its excellent performance. This study introduces a scalable and efficient method for OAPS synthesis, with significant potential for industrial applications in advanced materials and nanotechnology.
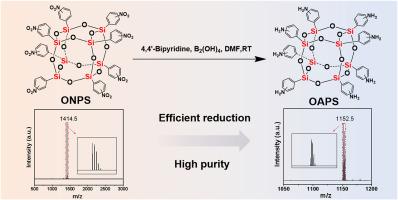
用B2(OH)4在室温下胺化八(硝基苯)硅氧烷合成八(氨基苯基)硅氧烷及表征
八氨基苯基硅氧烷(OAPS)是一种极具发展前景的多面体低聚硅氧烷(POSS)衍生物,具有独特的结构和功能特性,非常适合于高级材料的应用。本研究以四羟基二硼[B2(OH)4]为还原剂,4,4′-联吡啶为催化介质,开发了一种高效、可持续的OAPS合成方法。该策略通过无金属方法简化了合成过程,在温和条件下操作,同时实现了高产量,并展示了卓越的工艺稳定性。首先合成八硝基苯硅氧烷(ONPS)作为前体。通过对不同还原剂、催化剂及其数量的综合考察和评价,提出了一种新的还原策略,使硝基完全高效地转化为氨基。通过傅里叶变换红外光谱(FTIR)和核磁共振(NMR)对所得OAPS进行了表征,基质辅助激光解吸/电离飞行时间质谱(MALDI-TOF MS)通过分子量分析证实了其高纯度和结构完整性。还提出了一种由B2(OH)4和4,4 ' -联吡啶协同作用促进的还原过程的机制途径。合成的OAPS具有良好的热稳定性,在氮气条件下初始分解温度为430.2℃,在空气条件下初始分解温度为399.3℃,表现出优异的性能。本研究介绍了一种可扩展且高效的OAPS合成方法,在先进材料和纳米技术领域具有重要的工业应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


