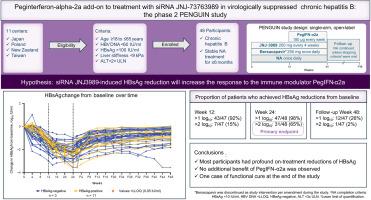
Peginterferon-alpha-2a add-on to treatment with siRNA JNJ-73763989 in virologically suppressed chronic hepatitis B: The phase II PENGUIN study
IF 7.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Previous studies showed that combination treatment with short interfering RNA JNJ-73763989 (JNJ-3989) ± capsid assembly modulator bersacapavir (JNJ-56136379) and nucleos(t)ide analogs (NAs) was well tolerated by patients with chronic HBV (CHB), with JNJ-3989 dose-dependent reductions in viral markers, including HBsAg. The open-label, single-arm phase IIa PENGUIN study (NCT04667104) evaluated this regimen plus pegylated interferon alpha-2a (PegIFN-α2a) in patients with virologically suppressed CHB.
Methods
Patients who were either HBeAg-positive or -negative virologically suppressed and taking NAs were included; all received JNJ-3989 ± bersacapavir for 24 weeks (some either did not start or discontinued bersacapavir as a result of protocol amendment) with PegIFN-α2a added during the final 12 weeks of treatment. The primary endpoint was the proportion of patients with ≥2 log10 IU/ml HBsAg reduction from baseline at week 24 (W24). Safety, tolerability, and proportion of patients meeting predefined NA stopping criteria were assessed. After 24 weeks of treatment, NA was stopped when stopping criteria were met; all patients were followed for 48 weeks. Efficacy and safety endpoints were summarized using descriptive statistics.
Results
In total, 48 patients (HBeAg positive, n = 11; HBeAg negative, n = 37) were enrolled; 47 reached follow-up week 48 (FUW48). Of the 48 patients, 31 (64.6%) achieved the primary endpoint; one achieved HBsAg seroclearance (<0.05 IU/ml) at both W24 and FUW48 after stopping NA. Mean HBsAg changes from baseline were -1.43, -2.18, and -0.71 log10IU/ml at W12, W24, and FUW48, respectively. At W24, 15/48 (31.3%) patients met NA stopping criteria; 11/15 (73.3%) remained off NA by FUW48. One patient achieved functional cure at FUW48. Adverse events were consistent with known PegIFN-α2a safety profiles.
Conclusions
Treatment with JNJ-3989 ± bersacapavir + NA and PegIFN-α2a was generally well tolerated. Most patients had pronounced HBsAg reductions from baseline, no additional benefit from PegIFN-α2a, and did not achieve functional cure.
Clinical Trials registration
The study is registered at ClinicalTrials.gov (NCT04667104).
Impact and implications
Previous studies with JNJ-73763989 (JNJ-3989) ± bersacapavir (JNJ-56136379) and nucleos(t)ide analogs (NAs) in patients with chronic hepatitis B (CHB) have demonstrated JNJ-3989 dose-dependent and robust reductions in viral markers; however, functional cure was only rarely achieved. The current study assessed whether the addition of pegylated interferon alpha-2a (PegIFN-α2a) to a JNJ-3989-based regimen after HBsAg levels were reduced would lead to further HBsAg declines and improved outcomes during off-treatment follow-up. Study results confirmed pronounced HBsAg reductions from baseline in virologically suppressed patients with HBeAg-positive or -negative CHB. However, the addition of PegIFN-α2a to JNJ-3989 ± bersacapavir and NA for the final 12 weeks of a 24-week treatment period did not appear to improve antiviral activity in the studied population. Studies in other CHB groups, including treatment-naïve, HBeAg-positive patients, could help elucidate whether this or other treatment regimens including JNJ-3989 and NA can lead to functional cure in a larger proportion of patients.
聚乙二醇干扰素- α -2a添加到siRNA JNJ-73763989治疗病毒学抑制的慢性乙型肝炎:II期企鹅研究
先前的研究表明,短干扰RNA JNJ-73763989 (JNJ-3989)±衣壳组装调节剂bersacapavir (JNJ-56136379)和核苷类似物(NAs)联合治疗慢性乙型肝炎(CHB)患者耐受性良好,JNJ-3989剂量依赖性降低病毒标志物,包括HBsAg。开放标签,单组IIa期PENGUIN研究(NCT04667104)评估了该方案与聚乙二醇化干扰素α -2a (PegIFN-α2a)在病毒学抑制的CHB患者中的应用。方法纳入hbeag阳性或阴性病毒学抑制并服用NAs的患者;所有患者均接受JNJ-3989±bersacapavir治疗24周(部分患者因方案修改未开始或停止使用bersacapavir),并在治疗的最后12周添加PegIFN-α2a。主要终点是第24周HBsAg较基线降低≥2 log10 IU/ml的患者比例(W24)。评估了安全性、耐受性和符合预定义NA停止标准的患者比例。治疗24周后,当满足停药标准时停止NA;所有患者随访48周。疗效和安全性终点采用描述性统计进行总结。结果共纳入48例患者(HBeAg阳性11例,HBeAg阴性37例);47例达到随访第48周(FUW48)。在48例患者中,31例(64.6%)达到了主要终点;1例患者在停用NA后,在W24和FUW48组均达到HBsAg血清清除率(0.05 IU/ml)。在W12、W24和FUW48时,与基线相比,平均HBsAg变化分别为-1.43、-2.18和-0.71 log10IU/ml。在W24时,15/48(31.3%)患者符合NA停止标准;11/15(73.3%)仍然远离NA。一名患者在FUW48达到功能性治愈。不良事件与已知的PegIFN-α2a安全性相符。结论JNJ-3989±bersacapavir + NA联合PegIFN-α2a治疗患者总体耐受良好。大多数患者的HBsAg较基线明显降低,PegIFN-α2a没有额外的益处,也没有实现功能性治愈。临床试验注册本研究已在ClinicalTrials.gov注册(NCT04667104)。影响和意义先前在慢性乙型肝炎(CHB)患者中使用JNJ-73763989 (JNJ-3989)±伯沙帕韦(JNJ-56136379)和核苷类似物(NAs)的研究表明,JNJ-3989对病毒标志物具有剂量依赖性和强效性降低;然而,功能性治愈很少实现。目前的研究评估了在HBsAg水平降低后,在基于jnj -3989的方案中加入聚乙二醇化干扰素α -2a (PegIFN-α2a)是否会导致HBsAg进一步下降,并在治疗后随访期间改善预后。研究结果证实,在hbeag阳性或阴性的CHB病毒学抑制患者中,HBsAg明显低于基线。然而,在24周治疗期的最后12周,在JNJ-3989±bersacapavir和NA中添加PegIFN-α2a似乎没有提高研究人群的抗病毒活性。对其他CHB组(包括treatment-naïve、hbeag阳性患者)的研究可以帮助阐明这种或其他治疗方案(包括JNJ-3989和NA)是否可以在更大比例的患者中实现功能性治愈。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


