Tubulin isotypes of C. elegans harness the mechanosensitivity of the lattice for microtubule luminal accessibility
IF 18.4
1区 物理与天体物理
Q1 PHYSICS, MULTIDISCIPLINARY
引用次数: 0
Abstract
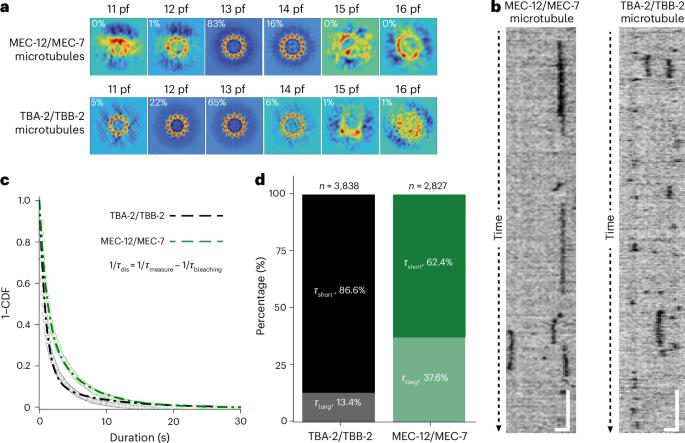
Microtubules are hollow cylindrical cytoskeletal polymers of laterally associated protofilaments that contain head-to-tail aligned ɑ/β-tubulin heterodimers. Although the exposed microtubule exterior is readily accessible to proteins, the mechanism governing the accessibility of the confined microtubule lumen to luminal particles remains unknown. Here we show that certain tubulin family proteins (isotypes) facilitate luminal accessibility because of the mechanical properties and lateral interactions that they confer to the microtubules. We characterized the microtubules reconstituted from defined compositions of Caenorhabditis elegans tubulin isotypes. These tubulin isotypes form microtubules with comparable protofilament numbers but different luminal accessibility. We further revealed the role of tubulin isotypes in regulating the strength of inter-protofilament lateral interactions, which determines luminal accessibility through the mechanosensitivity of reversible protofilament separation. Deformation of the microtubule lattice, which generates stresses exceeding the strength of the lateral interactions, creates gaps between adjacent protofilaments, enhancing the accessibility of the lumen. Together, our findings uncovered the tubulin isotype-dependent mechanical plasticity that confers force sensitivity to the microtubule lattice and modulates the energy barrier for luminal proteins to access the lumen. Proteins interact with both the exterior and interior of microtubules. Here the relationship between microtubule building blocks and the accessibility of the microtubule interior to proteins is clarified.
秀丽隐杆线虫的微管蛋白同型利用晶格的机械敏感性来实现微管腔的可达性
微管是中空的圆柱形细胞骨架聚合物,由横向相关的原丝组成,含有首尾对齐的微管蛋白异源二聚体。尽管暴露的微管外部很容易被蛋白质接近,但控制受限制的微管管腔对管腔颗粒的可及性的机制仍然未知。在这里,我们表明某些微管蛋白家族蛋白(同种型)由于其赋予微管的机械特性和横向相互作用而促进了腔内可达性。我们描述了从秀丽隐杆线虫微管蛋白同种型的定义组成重建的微管。这些微管蛋白同型形成的微管具有相当的原丝数量,但不同的管腔可及性。我们进一步揭示了微管蛋白同型在调节原丝间横向相互作用强度中的作用,这种相互作用通过可逆原丝分离的机械敏感性决定了腔内可及性。微管晶格的变形产生的应力超过了横向相互作用的强度,在相邻的原丝之间产生间隙,增强了管腔的可达性。总之,我们的发现揭示了微管蛋白同型依赖的机械可塑性,它赋予微管晶格力敏感性,并调节管腔蛋白进入管腔的能量屏障。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Physics
物理-物理:综合
CiteScore
30.40
自引率
2.00%
发文量
349
审稿时长
4-8 weeks
期刊介绍:
Nature Physics is dedicated to publishing top-tier original research in physics with a fair and rigorous review process. It provides high visibility and access to a broad readership, maintaining high standards in copy editing and production, ensuring rapid publication, and maintaining independence from academic societies and other vested interests.
The journal presents two main research paper formats: Letters and Articles. Alongside primary research, Nature Physics serves as a central source for valuable information within the physics community through Review Articles, News & Views, Research Highlights covering crucial developments across the physics literature, Commentaries, Book Reviews, and Correspondence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


