Is the N2 dissociative mechanism universal across stepped metal surfaces for ammonia synthesis?
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The need of more efficient catalysts for thermal ammonia synthesis is urgent as ever, but is limited by the absence of innovative mechanisms beyond traditional dissociative mechanism. Recently, however, the associative mechanism has emerged as a viable route especially in electro-catalysis, calling for a deeper understanding in its possibility in catalyst design for thermal process. Here, we conduct a comprehensive analysis of elementary reactions on stepped metal surfaces, establishing scaling relationships for the associative mechanism and refining the ammonia synthesis volcano plot. Our results reveal that under typical reaction conditions (473–773 K, 1–100 bar), metal surfaces such as Ru, Fe, and Rh predominantly follow the dissociative mechanism with high reaction rates, while Mo, Ni, Pd, and Cu favor the associative mechanism but exhibit lower reaction rates. Expanding these finding through volcano plots to 21 metal surfaces, we observed that pure metal surfaces do not fall into the high-activity region for associative hydrogenation due to the scaling relationships. Therefore, we identify the N2 direct hydrogenation step of N–HN bond formation as a key descriptor among the numerous elementary reaction steps of associative mechanism, and the efficiency of the associative pathway can be significantly enhanced by lowering the energy barrier of this step-particularly by breaking the linear scaling relationships. These findings challenge the traditional view of stepped metal surfaces favor dissociative mechanisms, and provide a new paradigm for catalysts design by reinventing reaction mechanisms.

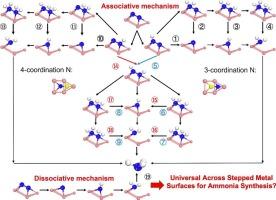
氨合成的N2解离机制在阶梯式金属表面上是通用的吗?
对高效热氨合成催化剂的需求一如既往地迫切,但由于缺乏超越传统解离机制的创新机制而受到限制。然而,近年来,缔合机制已成为一种可行的途径,特别是在电催化中,需要对其在热过程催化剂设计中的可能性有更深入的了解。在此,我们对阶梯金属表面上的元素反应进行了综合分析,建立了联想机理的标度关系,并完善了氨合成火山图。结果表明,在典型反应条件下(473-773 K, 1-100 bar), Ru、Fe和Rh等金属表面主要遵循离解机制,反应速率高,而Mo、Ni、Pd和Cu倾向于缔合机制,反应速率较低。将这些发现通过火山图扩展到21个金属表面,我们观察到纯金属表面由于结垢关系而不属于缔合氢化的高活性区域。因此,我们将N-HN键形成的N2直接加氢步骤确定为结合机理众多基本反应步骤中的关键描述符,并且通过降低该步骤的能垒,特别是通过打破线性标度关系,可以显著提高结合途径的效率。这些发现挑战了阶梯式金属表面有利于解离机制的传统观点,并通过重塑反应机制为催化剂设计提供了新的范式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


