Hydrodenitrogenation of alkylamines over supported Ru-based catalysts
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Hydrodenitrogenation (HDN) of 1-cyclohexylethylamine to ethylcyclohexane was investigated with various Ru-based catalysts. Ru-Ir/α-Al2O3 (1 wt% Ru, 4 wt% Ir) catalyst was found to be superior to other supported Ru catalysts in terms of activity and selectivity to ethylcyclohexane. Characterization with XRD, TEM-EDX and XAFS showed that uniform Ru-Ir alloy particles of approximately 4 nm in size were formed on α-Al2O3. The optimal Ru-Ir/α-Al2O3 catalyst gave 94 % yield of ethylcyclohexane. The time-course results and the reaction results of bis-(1-cyclohexylethyl)-amine (coupled compound of the substrate) demonstrated that the direct C![]() N cleavage route and indirect route via the coupled compound both proceeded in the HDN of 1-cyclohexylethylamine. The propylamine-TPD results with H2 treatment after the adsorption of propylamine on the catalyst suggested that the adsorption of H2 and amine was favorable on the Ru and Ir species, respectively. The catalyst was reusable after washing with ethanol with a slight loss of activity. The catalyst was also effective for the HDN reaction of other amines, and the HDN of some substrates proceeded mainly in the indirect route via the coupled compound.
N cleavage route and indirect route via the coupled compound both proceeded in the HDN of 1-cyclohexylethylamine. The propylamine-TPD results with H2 treatment after the adsorption of propylamine on the catalyst suggested that the adsorption of H2 and amine was favorable on the Ru and Ir species, respectively. The catalyst was reusable after washing with ethanol with a slight loss of activity. The catalyst was also effective for the HDN reaction of other amines, and the HDN of some substrates proceeded mainly in the indirect route via the coupled compound.
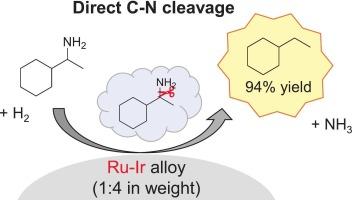
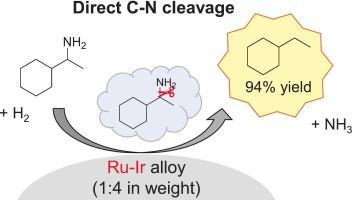
负载型钌基催化剂上烷基胺的加氢脱氮
用不同的钌基催化剂研究了1-环己基乙胺加氢脱氮制乙基环己烷的反应。RuIr/α-Al2O3(1 wt% Ru, 4 wt% Ir)催化剂对乙基环己烷的催化活性和选择性均优于其他负载Ru催化剂。XRD、TEM-EDX和XAFS表征表明,α-Al2O3表面形成了尺寸约为4 nm的均匀RuIr合金颗粒。最佳RuIr/α-Al2O3催化剂的乙基环己烷收率为94 %。时间过程结果和双-(1-环己基乙基)胺(底物偶联化合物)的反应结果表明,通过偶联化合物直接裂解CN的途径和通过偶联化合物间接裂解CN的途径均在1-环己基乙胺的HDN中进行。丙胺在催化剂上吸附后,H2处理后的丙胺- tpd结果表明,H2和胺对Ru和Ir的吸附分别有利。催化剂经乙醇洗涤后可重复使用,活性稍有损失。该催化剂对其他胺类的HDN反应也有效,部分底物的HDN反应主要通过偶联化合物间接进行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


