Genetics-nutrition interactions control diurnal enhancer-promoter dynamics and liver lipid metabolism
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The circadian clock controls 24-h rhythmic processes. However, how genetic variations outside clock genes impact peripheral diurnal rhythms remains largely unknown. Here, we find that genetic variation contributes to different diurnal patterns of hepatic gene expression in both humans and mice. Nutritional challenges alter the rhythmicity of gene expression in mouse liver in a strain-specific manner. Remarkably, genetics and nutrition interdependently control more than 80% of rhythmic gene and enhancer-promoter interactions (E-PIs), with a noncanonical clock regulator, estrogen-related receptor gamma (ESRRγ), emerging as a top transcription factor during motif mining. Knockout of Esrrγ abolishes strain-specific metabolic processes in response to diet in mice, while single-nucleotide polymorphisms (SNPs) associated with rhythmic gene expression are enriched in E-PIs in steatotic human livers and correlate with lipid metabolism traits. These findings reveal a previously underappreciated temporal aspect of genetics-environment interaction in regulating lipid metabolic traits, with implications for individual variations in obesity-associated disease susceptibility and personalized chronotherapy.
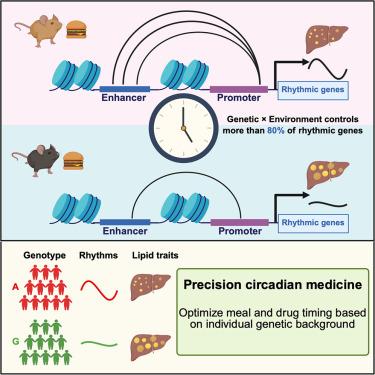
遗传-营养相互作用控制增强子-启动子动态和肝脏脂质代谢
生物钟控制着24小时的节律过程。然而,生物钟基因之外的遗传变异如何影响外周昼夜节律在很大程度上仍然未知。在这里,我们发现遗传变异有助于人类和小鼠肝脏基因表达的不同日模式。营养挑战以一种菌株特异性的方式改变小鼠肝脏基因表达的节律性。值得注意的是,遗传和营养相互依赖地控制着超过80%的节律性基因和增强子-启动子相互作用(e - pi),其中非规范时钟调节因子雌激素相关受体γ (ESRRγ)在基序挖掘过程中成为顶级转录因子。敲除Esrrγ可以消除小鼠对饮食的特异性代谢过程,而与节律性基因表达相关的单核苷酸多态性(snp)在脂肪变性人肝脏的e- pi中富集,并与脂质代谢特征相关。这些发现揭示了以前未被重视的遗传-环境相互作用在调节脂质代谢特征中的时间方面,对肥胖相关疾病易感性和个性化时间疗法的个体差异具有启示意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


