Enhancer remodeling by OTX2 directs specification and patterning of mammalian definitive endoderm
IF 8.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
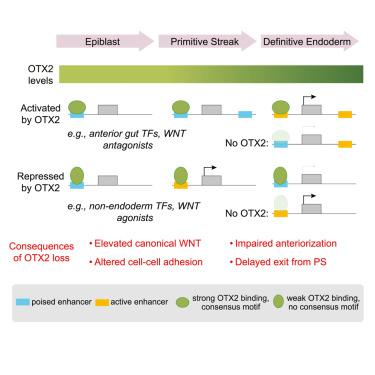
The molecular mechanisms that drive essential patterning events in the mammalian embryo remain poorly understood. Analysis of transcription factor expression kinetics at peri-gastrulation stages of development suggest Otx2 as a candidate regulator of the definitive endoderm, the precursor of all gut-derived organs. Accordingly, timed OTX2 depletion in gastruloids or during directed differentiation results in abnormal definitive endoderm specification in mouse and human, characterized by altered expression of components and transcriptional targets of the canonical WNT signaling pathway, perturbed adhesion and migration programs, and de-repression of regulators of other lineages. These defects cumulate in impaired foregut formation. Mechanistically, OTX2 is required to activate a subset of endoderm-specific enhancers and to suppress select enhancers of other lineages, allowing timely exit from the primitive streak and correct specification of anterior endoderm. Our results establish OTX2 as an early gut regulator and suggest molecular principles underlying spatiotemporal cell identity conserved across germ layers and species.
OTX2的增强子重塑指导哺乳动物终末内胚层的规范和模式
在哺乳动物胚胎中驱动基本模式事件的分子机制仍然知之甚少。转录因子在原肠胚周围发育阶段的表达动力学分析表明,Otx2是最终内胚层(所有肠源性器官的前体)的候选调节因子。因此,在类胃组织或定向分化过程中,OTX2的定时耗竭会导致小鼠和人类的最终内胚层特征异常,其特征是典型WNT信号通路组分和转录靶点的表达改变,粘附和迁移程序受到干扰,以及其他谱系调节因子的去抑制。这些缺陷累积在受损的前肠形成。从机制上讲,OTX2需要激活内胚层特异性增强子子集并抑制其他谱系的选择性增强子,从而允许及时退出原始条纹并正确指定前内胚层。我们的研究结果表明OTX2是早期肠道调节因子,并提示了跨胚层和物种的时空细胞特性保守的分子原理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


