Cryo-EM structures of human OAT1 reveal drug binding and inhibition mechanisms
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The organic anion transporter 1 (OAT1) plays a key role in excreting waste from organic drug metabolism and contributes significantly to drug-drug interactions and drug disposition. However, the structural basis of specific substrate and inhibitor transport by human OAT1 (hOAT1) has remained elusive. We determined four cryogenic electron microscopy (cryo-EM) structures of hOAT1 in its inward-facing conformation: the apo form, the substrate (olmesartan)-bound form with different anions, and the inhibitor (probenecid)-bound form. Structural and functional analyses revealed that Ser203 has an auxiliary role in chloride coordination, and it is a critical residue modulating olmesartan transport via chloride ion interactions. Structural comparisons indicate that inhibitors not only compete with substrates, but also obstruct substrate exit and entry from the cytoplasmic side, thereby increasing inhibitor retention. The findings can support drug development by providing insights into substrate recognition and the mechanism by which inhibitors arrest the OAT1 transport cycle.
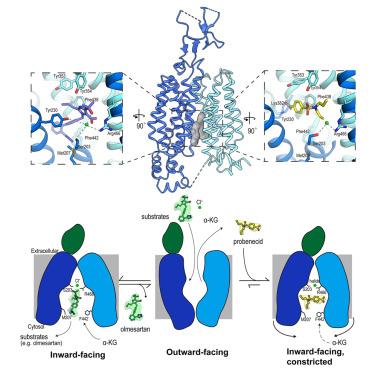
人OAT1的低温电镜结构揭示了药物结合和抑制机制
有机阴离子转运蛋白1 (ooat1)在有机药物代谢的废物排泄中起着关键作用,在药物-药物相互作用和药物处置中起着重要作用。然而,人类OAT1 (hOAT1)转运特异性底物和抑制剂的结构基础仍不清楚。我们在低温电子显微镜(cryo-EM)下确定了hOAT1的四种内向构象结构:载子形式,底物(奥美沙坦)与不同阴离子结合形式,以及抑制剂(苯丙酸)结合形式。结构和功能分析表明,Ser203在氯离子配位中具有辅助作用,是通过氯离子相互作用调节奥美沙坦转运的关键残基。结构比较表明,抑制剂不仅与底物竞争,而且还阻碍底物从细胞质侧进出,从而增加抑制剂的保留率。这些发现可以通过深入了解底物识别和抑制剂阻止OAT1转运周期的机制来支持药物开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


