CHIP protects lysosomes from CLN4 mutant-induced membrane damage
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
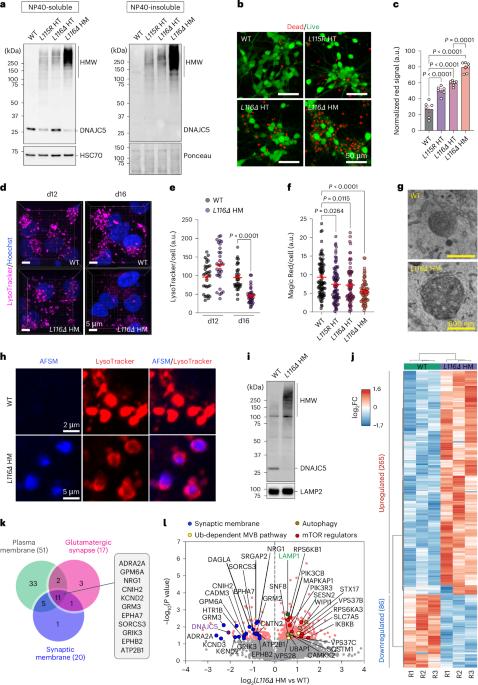
Understanding how cells mitigate lysosomal damage is critical for unravelling pathogenic mechanisms of lysosome-related diseases. Here we generate and characterize induced pluripotent stem cell (iPSC)-derived neurons (i3Neuron) bearing ceroid lipofuscinosis neuronal 4 (CLN4)-linked DNAJC5 mutations, which revealed extensive lysosomal abnormality in mutant neurons. In vitro membrane-damaging experiments establish lysosomal damages caused by lysosome-associated CLN4 mutant aggregates, as a critical pathogenic linchpin in CLN4-associated neurodegeneration. Intriguingly, in non-neuronal cells, a ubiquitin-dependent microautophagy mechanism downregulates CLN4 aggregates to counteract CLN4-associated lysotoxicity. Genome-wide CRISPR screens identify the ubiquitin ligase carboxyl terminus of Hsc70-interacting protein (CHIP) as a central microautophagy regulator that confers ubiquitin-dependent lysosome protection. Importantly, CHIP’s lysosome protection function is transferrable: ectopic CHIP improves lysosomal function in CLN4 i3Neurons and effectively alleviates lipofuscin accumulation and cell death in a Drosophila CLN4 disease model. Our study establishes CHIP-mediated microautophagy as a key organelle guardian that preserves lysosome integrity, offering new insights into therapeutic development for lysosome-related neurodegenerative diseases. Lee et al. use an aggregation-prone CLN4 mutant that causes lysosomal damage in neurons and show that in non-neurons, the ubiquitin ligase CHIP prevents CLN4-dependent lysotoxicity via microautophagy.
CHIP保护溶酶体免受CLN4突变体诱导的膜损伤
了解细胞如何减轻溶酶体损伤对于揭示溶酶体相关疾病的致病机制至关重要。在这里,我们生成并表征了诱导多能干细胞(iPSC)衍生的神经元(i3Neuron)携带ceroid lipofuscinosis neuronal 4 (CLN4)相关的DNAJC5突变,这表明突变神经元中存在广泛的溶酶体异常。体外膜损伤实验证实,溶酶体相关CLN4突变聚集体引起的溶酶体损伤是CLN4相关神经退行性变的关键致病关键。有趣的是,在非神经元细胞中,泛素依赖的微自噬机制下调CLN4聚集以抵消CLN4相关的溶酶毒性。全基因组CRISPR筛选鉴定出hsc70相互作用蛋白(CHIP)的泛素连接酶羧基端是一个中心微自噬调节因子,赋予泛素依赖性溶酶体保护。重要的是,CHIP的溶酶体保护功能是可转移的:在果蝇CLN4疾病模型中,异位CHIP改善了CLN4 i3神经元中的溶酶体功能,并有效缓解了脂褐素积累和细胞死亡。我们的研究证实了chip介导的微自噬是保持溶酶体完整性的关键细胞器守护者,为溶酶体相关神经退行性疾病的治疗发展提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


