Transient gene melting governs the timing of oligodendrocyte maturation
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Cellular maturation is a crucial step for tissue formation and function, distinct from the initial steps of differentiation and cell fate specification. In the central nervous system, failure of oligodendrocyte maturation is linked to diseases such as multiple sclerosis. Here, we report a transcriptional mechanism that governs the timing of oligodendrocyte maturation. After progenitor cells differentiate into immature oligodendrocytes, the transcription factor SOX6 redistributes from super-enhancers to cluster across specific gene bodies. These sites exhibit extensive chromatin decondensation and transcription, which abruptly turn off upon maturation. Suppression of SOX6 deactivates these immaturity loci, accelerating the transition to mature, myelinating oligodendrocytes. Notably, cells harboring this immature SOX6 gene signature are enriched in multiple sclerosis patient brains and antisense oligonucleotide-mediated Sox6 knockdown drives oligodendrocyte maturation in mice. Our findings establish SOX6 as a key regulator of oligodendrocyte maturation and highlight its potential as a therapeutic target to promote myelination in disease.
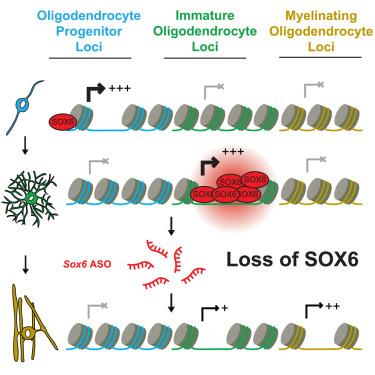
瞬时基因融化决定了少突胶质细胞成熟的时间
细胞成熟是组织形成和功能的关键步骤,不同于分化和细胞命运规范的初始步骤。在中枢神经系统中,少突胶质细胞成熟失败与多发性硬化症等疾病有关。在这里,我们报告了一种调控少突胶质细胞成熟时间的转录机制。祖细胞分化为未成熟的少突胶质细胞后,转录因子SOX6从超增强子重新分布到特定基因体的聚集。这些位点表现出广泛的染色质去浓缩和转录,在成熟时突然关闭。抑制SOX6使这些不成熟的基因座失活,加速向成熟的髓鞘少突胶质细胞的过渡。值得注意的是,含有这种未成熟SOX6基因标记的细胞在多发性硬化症患者的大脑中富集,反义寡核苷酸介导的SOX6敲低驱动小鼠少突胶质细胞成熟。我们的研究结果确定SOX6是少突胶质细胞成熟的关键调节因子,并强调其作为促进疾病髓鞘形成的治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


