Human monoclonal antibodies targeting A35 protect from death caused by mpox
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The 2022 mpox outbreak highlighted the serious threat of monkeypox virus (MPXV), yet effective treatments are lacking. From an mpox-convalescent individual, we identified three high-affinity human monoclonal antibodies (mAbs) (named EV35-2, EV35-6, and EV35-7) that target the A35 protein in MPXV. These antibodies block viral spread in vitro and protect mice against lethal MPXV and vaccinia virus infection via both Fc-dependent and independent mechanisms. Levels of serum antibodies targeting the same epitopes are increased in mpox-convalescent humans, and higher levels of these antibodies in the sera are linked to shorter symptom duration and no hospitalization. Systems-level multivariate analysis indicated that mpox-convalescent serum antibodies targeting the same epitopic region as these three mAbs may function cooperatively, with additive associations to clinical protection. Two of the antibodies use a conserved IGHD2-21-encoded CxGGDCx motif in their CDRH3 region to bind a highly conserved poxvirus epitope. These findings establish A35 as a critical therapeutic target and highlight A35-specific mAbs as promising candidates for next-generation orthopoxvirus treatments.
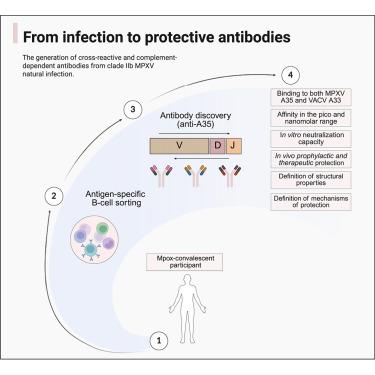
针对A35的人单克隆抗体可防止m痘引起的死亡
2022年猴痘疫情凸显了猴痘病毒(MPXV)的严重威胁,但缺乏有效的治疗方法。从一名mpox恢复期个体中,我们鉴定出三种高亲和力的人单克隆抗体(mab)(命名为EV35-2、EV35-6和EV35-7),它们靶向MPXV中的A35蛋白。这些抗体阻断病毒在体外的传播,并通过fc依赖性和独立机制保护小鼠免受致命的MPXV和牛痘病毒感染。针对相同表位的血清抗体水平在mpox恢复期的人中升高,血清中这些抗体水平升高与症状持续时间缩短和不住院有关。系统水平的多变量分析表明,针对相同表位区域的mpox-恢复期血清抗体与这三种单克隆抗体可能协同起作用,与临床保护具有附加关联。其中两种抗体在其CDRH3区域使用保守的ighd2 -21编码的CxGGDCx基序结合高度保守的痘病毒表位。这些发现确立了A35是一个关键的治疗靶点,并强调了A35特异性单克隆抗体是下一代正痘病毒治疗的有希望的候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


