Structural basis of poxvirus fusion regulation and anti-A16/G9 antibody-mediated neutralization and protection
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
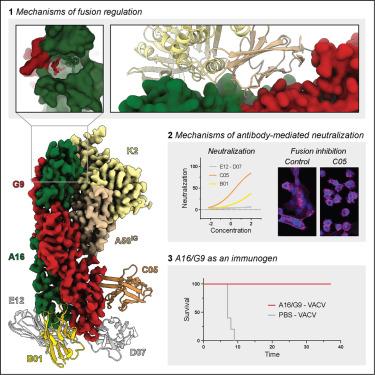
Monkeypox virus (MPXV) is a poxvirus endemic to Central and West Africa with high epidemic potential. Poxviruses enter host cells via a conserved entry-fusion complex (EFC), which mediates viral fusion to the cell membrane. The EFC is a promising therapeutic target, but the absence of structural data has limited the development of fusion-inhibiting treatments. Here, we investigated A16/G9, a subcomplex of the EFC that controls fusion timing. Using cryo-electron microscopy, we showed how A16/G9 interacts with A56/K2, a viral fusion suppressor that prevents superinfection. Immunization with A16/G9 elicited a protective immune response in mice. Using X-ray crystallography, we characterized two neutralizing antibodies and engineered a chimeric antibody that cross-neutralizes several poxviruses more efficiently than 7D11, the most potent antibody targeting the EFC described to date. These findings highlight the potential of A16/G9 as a candidate for subunit vaccines and identify regions of the EFC as targets for antiviral development.
痘病毒融合调控及抗a16 /G9抗体介导的中和和保护的结构基础
猴痘病毒(MPXV)是中非和西非的一种痘病毒,具有很高的流行潜力。痘病毒通过保守的入口融合复合体(EFC)进入宿主细胞,介导病毒与细胞膜的融合。EFC是一个很有前景的治疗靶点,但缺乏结构数据限制了融合抑制治疗的发展。在这里,我们研究了控制融合时间的EFC亚复合物A16/G9。利用低温电子显微镜,我们展示了A16/G9如何与A56/K2相互作用,A56/K2是一种防止重复感染的病毒融合抑制因子。用A16/G9免疫小鼠可引起保护性免疫反应。利用x射线晶体学,我们对两种中和抗体进行了表征,并设计了一种嵌合抗体,该抗体比7D11更有效地交叉中和几种痘病毒,7D11是迄今为止描述的针对EFC的最有效的抗体。这些发现突出了A16/G9作为亚单位疫苗候选的潜力,并确定了EFC区域作为抗病毒开发的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


