Ume6 protein complexes connect morphogenesis, adherence and hypoxic genes to shape Candida albicans biofilm architecture
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
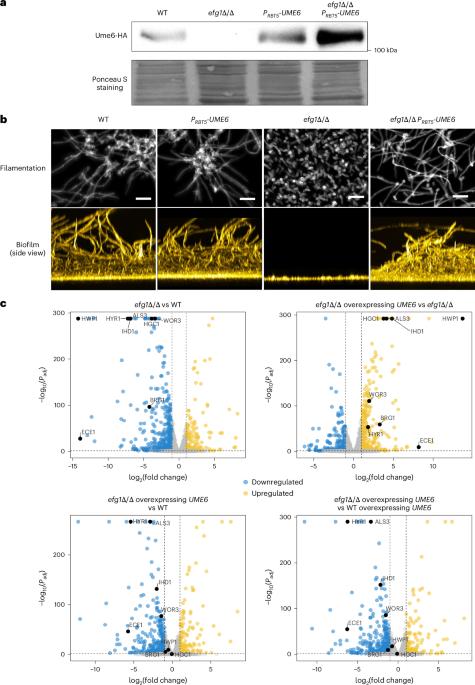
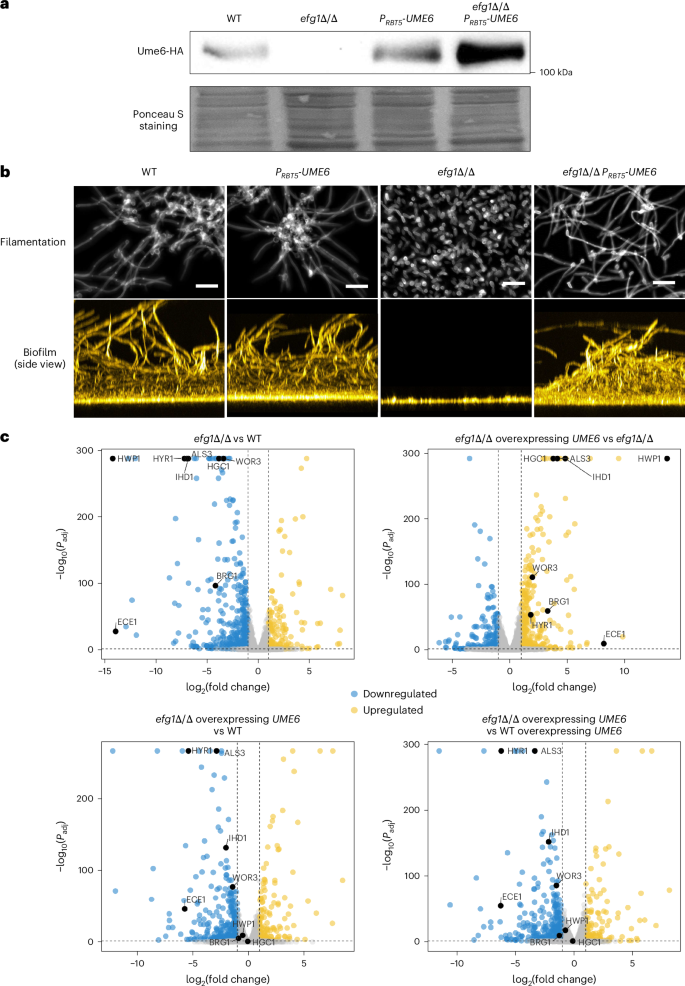
Biofilms of the fungal pathogen Candida albicans can form on implanted medical devices and contribute to fungal virulence and are recalcitrant to antifungal therapy. The transcription factor Ume6 directs hyphal cell elongation and thus promotes biofilm formation in C. albicans. However, how exactly this key biofilm and virulence regulator functions has remained unclear. Here RNA sequencing and chromatin immunoprecipitation with sequencing data show that Ume6 binds to and activates multiple biofilm-relevant genes. Ume6-associated sequence motifs correspond to binding sites for biofilm master regulators Efg1 and Ndt80, and hypoxic response regulator Upc2. Co-immunoprecipitation assays show the existence of Ume6–Efg1, Ume6–Ndt80 and Ume6–Upc2 protein complexes. Promoter binding of Ume6 is partially dependent upon Efg1, Ndt80 or Upc2, as is Ume6 target gene activation, thus indicating that the protein complexes function to drive Ume6–target gene interaction. Ume6 therefore acts as a bridge that connects the hyphal morphogenesis and adherence genes that shape biofilm architecture and the hypoxic response genes required for growth in the low-oxygen biofilm environment. These findings are vital for our understanding of the pathobiology of C. albicans and could open the way to new treatment options. Complexes of the transcription factor Ume6 bridge morphogenesis, adherence and hypoxic genes that determine biofilm architecture in the human fungal pathogen Candida albicans.
Ume6蛋白复合物连接形态发生、粘附和缺氧基因,形成白色念珠菌生物膜结构
真菌病原体白色念珠菌的生物膜可以在植入的医疗器械上形成,并有助于真菌毒力,并且难以抗真菌治疗。转录因子Ume6指导菌丝细胞伸长,从而促进白色念珠菌生物膜的形成。然而,这种关键的生物膜和毒力调节剂究竟是如何起作用的仍不清楚。RNA测序和染色质免疫沉淀测序数据显示,Ume6结合并激活了多个生物膜相关基因。ume6相关序列基序对应于生物膜主调控因子Efg1和Ndt80以及缺氧反应调控因子Upc2的结合位点。共免疫沉淀实验显示存在Ume6-Efg1、Ume6-Ndt80和Ume6-Upc2蛋白复合物。Ume6启动子的结合部分依赖于Efg1、Ndt80或Upc2, Ume6靶基因的激活也是如此,这表明蛋白质复合物的功能是驱动Ume6靶基因的相互作用。因此,Ume6作为连接菌丝形态发生和粘附基因(形成生物膜结构)与低氧生物膜环境中生长所需的缺氧反应基因的桥梁。这些发现对我们了解白色念珠菌的病理生物学至关重要,并可能为新的治疗方案开辟道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


