SARS-CoV-2 infection induces pro-fibrotic and pro-thrombotic foam cell formation
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
COVID-19 and long COVID are characterized by a dysregulated immune response. However, the role of macrophages during viral infection is poorly defined. Here we demonstrate that SARS-CoV-2 infection results in increased macrophage numbers and extensive formation of enlarged lipid-laden macrophages or foam cells using humanized mice, rhesus macaques and post-mortem human lung tissue. Notably, infection by other coronaviruses tested, SARS-CoV-1, MERS-CoV and two bat coronaviruses (SHC014-CoV or WIV1-CoV), did not result in macrophage proliferation or foam cell formation. Foam cells in SARS-CoV-2-infected human lung tissue display a pro-fibrotic and pro-thrombotic phenotype as they are enriched for genes associated with platelet activation and aggregation, as well as extracellular matrix organization and collagen synthesis. After viral clearance, macrophage numbers remain elevated, and lung fibrosis and thrombi persist. Importantly, we show that pre-exposure prophylaxis or early treatment with a SARS-CoV-2 antiviral, EIDD-2801, prevents increases in macrophage cell numbers and foam cell formation, and reduces fibrosis markers. These observations highlight the contribution of macrophages to lung inflammation and tissue injury leading to the pulmonary fibrosis observed in COVID-19 patients. SARS-CoV-2 infection can lead to sustained increased macrophage numbers and formation of enlarged lipid-laden macrophages after viral clearance. This can be prevented with antiviral treatment in mice.

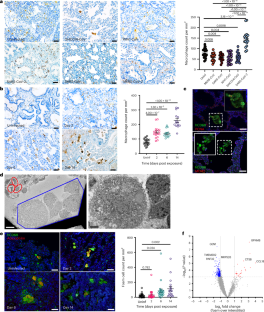
SARS-CoV-2感染诱导促纤维化和促血栓形成的泡沫细胞形成
COVID-19和长冠状病毒病的特征是免疫反应失调。然而,巨噬细胞在病毒感染中的作用尚不明确。在这里,我们通过人源化小鼠、恒河猴和死后的人肺组织证明,SARS-CoV-2感染导致巨噬细胞数量增加,并广泛形成增大的脂质巨噬细胞或泡沫细胞。值得注意的是,被测试的其他冠状病毒感染,SARS-CoV-1、MERS-CoV和两种蝙蝠冠状病毒(SHC014-CoV或WIV1-CoV),没有导致巨噬细胞增殖或泡沫细胞形成。sars - cov -2感染的人肺组织中的泡沫细胞表现出促纤维化和促血栓形成的表型,因为它们富含与血小板活化和聚集、细胞外基质组织和胶原合成相关的基因。病毒清除后,巨噬细胞数量仍然升高,肺纤维化和血栓持续存在。重要的是,我们发现暴露前预防或早期治疗SARS-CoV-2抗病毒药物EIDD-2801可防止巨噬细胞数量增加和泡沫细胞形成,并减少纤维化标志物。这些观察结果强调了巨噬细胞在COVID-19患者中观察到的肺部炎症和组织损伤导致肺纤维化的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


