Synthesis of Oxasilolanes by TBAT-Catalyzed Hydroxyl-Directed Hydrosilylation
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The reaction of allylic and homoallylic styrenyl alcohols with diphenylsilane and catalytic tetrabutylammonium difluorotriphenylsilicate (TBAT) produces 5- and 6-membered ring oxasilolanes, respectively. Differing substitution at the carbinol position, phenyl ring, and carbon–carbon double bond were all found to have significant impacts on both yield and diastereoselectivity. A mechanism is proposed involving fluoride-promoted intramolecular hydrosilylation and formation of an intermediate benzylic anion, followed by cyclization and oxasilolane formation. This mechanism is supported by double bond stereospecificity experiments along with the scope and stereochemical outcome of the reaction.
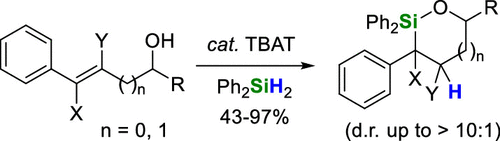
tbat催化羟基定向硅氢化反应合成草硅烷
烯丙基苯乙烯醇和均烯丙基苯乙烯醇与二苯基硅烷和催化四丁基二氟三苯基硅酸铵(TBAT)反应分别生成5元和6元环草硅烷。甲醇位置、苯环和碳碳双键的不同取代对产率和非对映选择性均有显著影响。提出了一种机制,涉及氟化物促进的分子内硅氢化和中间苯基阴离子的形成,然后是环化和草硅烷的形成。这一机制得到了双键立体特异性实验以及反应范围和立体化学结果的支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


