The dark side of the soil carbon cycle: Hydroxyl radicals and abiotic CO2 production
IF 10.3
1区 农林科学
Q1 SOIL SCIENCE
引用次数: 0
Abstract
Fenton-type reactions without light (Dark-Fenton) in some forest soils generate hydroxyl radicals (•OH) from ferrous iron [Fe(II)] and dissolved organic carbon (DOC) under fluctuating anoxic–oxic conditions. We hypothesized that Fe(II) concentrated in micropores (<10 μm) raises radical production in soil, exceeding electron donation solely by DOC, and that radical-mediated abiotic oxidation releases CO2. Four undisturbed humid forest soils, ranging from sandy loam to silty clay loam with contrasting parent materials, were incubated anoxically (∼14 days) and then exposed to oxygen for 24 h in the dark. We introduced hydrogen peroxide (5–300 μM), and the δ13C signature confirmed that the CO2 originated from DOC rather than from bulk soil organic matter (SOM). Soils with higher Fe(II) (∼35 μM) in clay-rich or metamorphic parent materials produced up to ∼25 nM •OH in 24 h and released ∼20–25 % additional CO2 upon short-term re-oxygenation. Volcanic soils with ∼15 μM Fe(II) generated fewer radicals (∼5–10 nM) and only 5–10 % extra CO2. Micropores concentrated Fe(II), intensifying •OH formation and drove an abiotic CO2 flux that reached 25 % of total soil respiration. We condensed this effect into a single coefficient, ready for implementation in soil carbon models. Concluding, short redox pulses can oxidize 5–20 % of DOC via hydroxyl radicals produced by Fe(II) oxidation, adding a non-microbial (abiotic) flux to the total CO2 released from soil. These results revise the common view that soil CO2 originates exclusively from microbial and root respiration by revealing a sizeable abiotic contribution under fluctuating redox conditions.
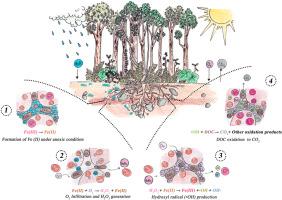
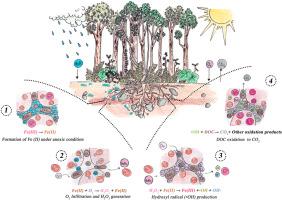
土壤碳循环的阴暗面:羟基自由基和非生物二氧化碳的产生
在一些森林土壤中,无光照的芬顿型反应(Dark-Fenton)在波动缺氧-缺氧条件下从亚铁[Fe(II)]和溶解有机碳(DOC)中产生羟基自由基(•OH)。我们假设在微孔(< 10 μm)中富集的Fe(II)提高了土壤中自由基的产量,超过了DOC单独给电子量,并且自由基介导的非生物氧化释放CO2。四种未受干扰的潮湿森林土壤,从砂质壤土到粉质粘土壤土,具有不同的母质,在缺氧条件下孵育(~ 14天),然后在黑暗中暴露于氧气中24小时。引入过氧化氢(5 ~ 300 μM), δ13C特征证实CO2来源于DOC而非土壤有机质(SOM)。富粘土或变质母质中Fe(II) (~ 35 μM)较高的土壤在24小时内产生高达~ 25 nM•OH,并在短期再氧化后释放出~ 20 - 25%的额外CO2。含~ 15 μM Fe(II)的火山土产生较少的自由基(~ 5-10 nM),仅产生5 - 10%的额外CO2。微孔富集了Fe(II),强化了•OH的形成,并驱动了非生物CO2通量,达到土壤呼吸总量的25%。我们将这个效应浓缩成一个系数,准备在土壤碳模型中实现。因此,短的氧化还原脉冲可以通过Fe(II)氧化产生的羟基自由基氧化5-20%的DOC,增加了土壤释放的总CO2的非微生物通量。这些结果通过揭示波动氧化还原条件下相当大的非生物贡献,修正了土壤二氧化碳完全来自微生物和根呼吸的普遍观点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Soil Biology & Biochemistry
农林科学-土壤科学
CiteScore
16.90
自引率
9.30%
发文量
312
审稿时长
49 days
期刊介绍:
Soil Biology & Biochemistry publishes original research articles of international significance focusing on biological processes in soil and their applications to soil and environmental quality. Major topics include the ecology and biochemical processes of soil organisms, their effects on the environment, and interactions with plants. The journal also welcomes state-of-the-art reviews and discussions on contemporary research in soil biology and biochemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


